Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
November 22, 2017
Sponsored Content
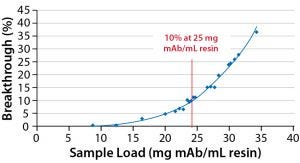
Figure 1: Results from testing of DBC of MabSelect SuRe resin
Biosimilars represent an innovative solution that can benefit both patients and healthcare systems by reducing the burden of rising treatment costs. To improve the availability, price, and access of medicines, many countries are implementing strategies to establish their own production capacity. To support such development, MAbxience (a Spanish biotechnology company specialized in research, development, and manufacture of biosimilar drugs) is committed to provide the manufacturing of high-quality products and processes that meet regulatory and technical requirements in all countries where it operates, using cutting edge single-use technology. Currently, MAbxience has sales contracts in more than 70 countries.
One of the biosimilar specialties present in MAbxience pipeline constitutes an Fc-fusion protein, with a molecular weight of Mr 150,000 and an isoelectric point of <5, for which a first-generation process was established by a third-party contract manufacturing organization (CMO). The molecule exhibits monoclonal antibody (MAb) behavior, but with a challenging glycosylation profile and complex tertiary and quaternary structures. Consequently, low product recovery and purity were obtained in the first-generation purification process. In addition, the purification protocols were poorly suited for manufacturing scale.
With the aim of improving the first-generation process to reach the manufacturability and purity required to produce material for phase 1 clinical trials, MAbxience contacted GE Healthcare’s Fast Trak Services team to initiate a collaborative project. This case study demonstrates the optimization of the downstream purification process to improve product purity and recovery of the biosimilar Fc-fusion protein. The optimization work was conducted by Fast Trak scientists. Process optimization focused on improving the affinity chromatography (AF) capture step as well as the intermediate purification and polishing steps — using hydrophobic-interaction chromatography (HIC) and anion-exchange chromatography (AIEX), respectively — keeping the same process materials. In addition, a purification scheme more suitable for manufacturing scale was to be established.
Optimization of the Capture Step
The goal was to improve dynamic binding capacity (DBC) of the chromatography resin used in the capture step while maintaining or improving yield. A comparison of several protein A affinity resins was conducted, from which the MabSelect SuRe™ resin was selected. MabSelect SuRe resin was developed for process-scale MAb capture. The resin is designed with an alkali- and protease-stabilized, recombinant protein A ligand coupled to a rigid, high-flow agarose base matrix. The stability of the protein A ligand minimizes ligand leakage and allows for the use of rigorous and cost-effective cleaning procedures that include sodium hydroxide (NaOH). The highly cross-linked agarose base matrix of the resin enables the use of high flow velocities at manufacturing scale.
The DBC of the MabSelect SuRe resin was tested under the optimized conditions, and the results showed a 10% breakthrough at 24 mg MAb/L resin (Fig 1), a 100% improvement from the first-generation process.
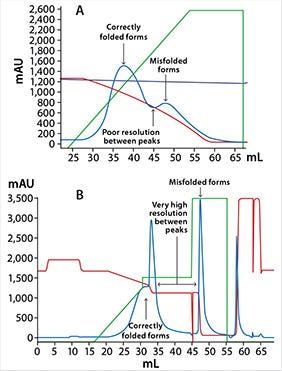
Figure 2: Chromatograms from intermediate purification step of (A) the original process and (B) the optimized process
Optimization of the intermediate Purification Step
The aim of this step is to remove misfolded versions of the target molecule. However, the first-generation process offered poor resolution between correctly folded and misfolded target (Figure 2A). The optimization goal for the intermediate purification step was to improve not only manufacturability, but also product purity. Although a preliminary screening of alternative resins from GE found two candidate resins that offered significantly better resolution, implementing a new resin was not possible with the limited time before project delivery. Hence, the work focused on optimizing process conditions for the current resin.
Optimization of loading conditions and changing from gradient to stepwise elution resulted in improved resolution, generating increased product recovery from 30–40% to ~50% and improved product purity (Figure 2B). However, the resin resolution was still too poor to remove impurities eluting at the front of the peak. Consequently, a partial gradient was still needed at the elution start to obtain the required product purity. Analysis of the purified protein by surface plasmon resonance (SPR) using a Biacore™ instrument verified a similar behavior of the target molecule to that of the originator molecule.
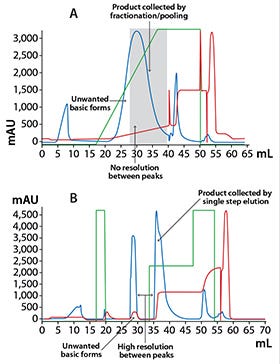
Figure 3: Chromatograms from the polishing step of (A) the original process and (B) the optimized process
Optimization of the Polishing Step
Q Sepharose™ Fast Flow anion-exchange resin was used in the polishing step. The resin was developed for industrial downstream processes and exhibits a high chemical stability, allowing for the use of well-proven cleaning-in-place (CIP) and sanitization protocols. The hydrophilic nature of the base matrix ensures low levels of nonspecific binding, leading to low levels of host-cell–derived impurities in the elution pool.
The aim of this step was to reduce charge variants to match those of the originator molecule. The first-generation process offered poor resolution, resulting in undesired basic charge variants in the product peak (Figure 3A). By optimizing loading conditions and changing from gradient to step-wise elution, resolution and product purity could be improved (Figure 3B). Also, the step yield could be increased from 60–70% to ~90% using the optimized protocol. Analysis of the purified protein by SPR using a Biacore instrument verified a similar behavior of the target molecule to the originator molecule.
Confirmation Runs
The final optimized process conditions were confirmed in three consistency batches at 0.5-L scale. Manufacturing batch record and solution record documents were prepared by the Fast Trak Services team, and the results showed reproducibility in yield and purity between the runs. Based on these results, technology transfer documentation was prepared.
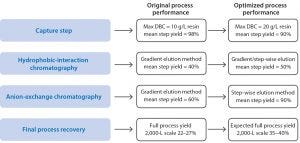
Figure 4: Improvements from the original process, using the optimized process
Process Summary and Discussion
Process improvements from the original process using the optimized process, are listed in Figure 4. A final report was delivered in accordance with set timelines, and the documentation required for technology transfer was prepared by the Fast Trak Services team. To facilitate technology transfer, Fast Trak scientists worked directly with a third-party CMO. By defining process-critical parameters in a simulation of conditions of the scaled-up process, the optimized process was successfully scaled to 500 L and 2,000 L for cGMP clinical manufacturing.
Conclusion
Collaborative projects can help ease risk and cost burdens while increasing speed to market. This case study demonstrates the optimization of a downstream purification of a MAb biosimilar as a collaboration between MAbxience and GE Healthcare’s Fast Trak Services team. Through resin selection and optimization of loading and elution conditions, product recovery, manufacturability, and purity were greatly improved. DBC of the resin used in the initial capture step was doubled, and recovery was significantly increased over the subsequent intermediate purification and polishing steps.
Acknowledgment
We thank MAbxience in Spain for kindly providing us with permission to use this body of work as a demonstration of our Fast Trak Services.
“To speed up the process optimization of the described biosimilar product, collaboration with GE Healthcare’s Fast Trak Services team was crucial for MAbxience. Thanks to the strong expertise and commitment of the Fast Trak team to protein downstream processing, the work was carried out smoothly to achieve the expected results according to the agreed timeline. Due to the valuable outcomes of this synergy, MAbxience will continue to collaborate with GE Healthcare’s Fast Trak Services team on other biosimilar projects.”
—Vincenzo Rivieccio (R&D Specialist, MAbxience, Spain)
You May Also Like