Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Sponsored by Refeyn

Photo 1: The SamuxMP system for mass photometric analysis of adenoassociated virus (AAV) vectors for gene therapies.
Current production processes for gene therapies based on adenoassociated virus (AAV) vectors generate many empty capsids. That problem complicates vector purification and diminishes product safety and quality. In a June 2022 webinar, Gareth Rogers (product manager at Refeyn Ltd.) observed that developers could benefit significantly from analytical instruments that assess empty-to-full (E:F) capsid ratios rapidly. He explained how the SamuxMP mass photometry system (Photo 1) could address such needs. Kirsty McManus (senior scientist in AAV characterization at Pharmaron Gene Therapy) described how her company, a contract development and manufacturing organization (CDMO), has used the instrument to facilitate optimization during downstream process development.
Rogers’s Presentation
Rogers highlighted several difficulties associated with the large number of empty capsids produced during AAV cultivation. High percentages of empty capsids increase burdens on downstream purification steps, increasing cost of goods (CoG) in turn. Such particles also pose problems for patients. High percentages of nonfunctional AAVs reduce both target viral-genome concentrations and transduction efficiency, limiting therapeutic benefit. Empty capsids also raise immunogenicity risks. Thus, gene therapy manufacturers must optimize downstream purification steps to help ensure product efficacy, safety, and quality. Rogers noted, however, that process development scientists have lacked analytical capabilities for rapid assessment of E:F capsid ratios.
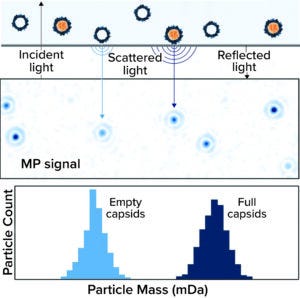
Figure 1: Mass photometry (MP) measures the light reflected by a glass coverslip, the light scattered by particles within a sample, and the interference between those values to determine a particle’s mass. That principle enables calculation of empty-to-full capsid ratios during characterization of adenoassociated virus (AAV) vectors.
Mass photometry (MP) simply, accurately, and quickly distinguishes between full and empty AAV capsids. In a typical workflow, samples are placed onto a glass coverslip and then exposed to light. Some light reflects from the glass–water interface; some passes through and is scattered by particles in the sample. Mass photometers measure the amounts of reflected and scattered light as well as the level of interference between them to determine each particle’s mass (Figure 1). Contaminants, empty capsids, and full capsids differ significantly in mass — <2 mDa, ~3.7 mDa, and ~4.1–5.3 mDa, respectively. Thus, analyzing the mass distribution within a sample enables determination of E:F capsid ratios.
Rogers noted that the SamuxMP benchtop MP system is optimized for a mass range relevant to AAV analytics (3–6 mDa). Made of materials chosen to simplify decontamination procedures, the instrument comes preloaded with software designed to facilitate calculation of E:F capsid ratios. Rogers highlighted that the system can handle low-volume samples (10–20 µL) from any downstream process step. Because MP is a label-free method, samples require minimal preparation. Results are generated within five minutes. Such features, Rogers explained, could make the SamuxMP system an attractive orthogonal technology to time- and resource-intensive analytical centrifugation (AUC), which is the industry’s gold-standard method for quantifying E:F capsid ratios.
The SamuxMP system takes accurate measurements across a broad range of E:F capsid ratios. Rogers related that his company collaborated with the Cell and Gene Therapy Catapult (London, UK) to evaluate the performance of a prototype. Catapult scientists mixed reference materials of empty and full capsids in different proportions, then ran those samples on the prototype. Observed E:F capsid ratios accorded with expected values. The results also tracked closely with historical data from tests based on AUC and transmission electron cryomicroscopy (cryo-TEM).
The Catapult conducted additional studies for robustness assessment. Two operators performed six replicate experiments based on six independent runs. Data from those tests demonstrated that the system measures samples with high intermediate precision and experimental reproducibility, demonstrated by coefficients of variation (CVs) of 11.74% and 10.87%, respectively. Both of those values, Rogers noted, were well within the Catapult’s acceptance criteria.
Refeyn later worked with Utrecht University to study the effects of different AAV serotypes on SamuxMP system performance. Scientists compared mass-distribution profiles across AAV8 samples from two distributors and AAV5 samples from one supplier. The profiles were highly similar, indicating not only that the AAV8 data were precise and reproducible, but also that the instrument works across serotypes.
McManus’s Presentation
Pharmaron Gene Therapy provides CDMO services for AAV-based therapies. McManus noted that E:F capsid analysis is among the most difficult kinds of testing that her company performs. To help clients make informed decisions during process development, Pharmaron needs methods that perform robustly and rapidly using relatively inexpensive materials. Such methods also must be able to handle small-volume samples that might have low vector concentrations.
Pharmaron evaluated the SamuxMP system to determine whether it could meet those criteria. Scientists compared its performance with that of an AUC system in analyzing samples of drug-substance purity. The instruments generated comparable results that resolved empty from partial capsids, so analysts performed similar studies with samples based on a different AAV serotype. Results from those experiments also tracked closely across instruments, supporting the SamuxMP system’s application to complement AUC. Equally encouraging was that the MP instrument resolved empty, full, and partially filled capsids of that serotype.
Next, McManus’s team performed experiments like those of the Cell and Gene Therapy Catapult. Reference materials for full/partial and empty capsids were blended in different proportions, then analyzed using a SamuxMP instrument. Based on data from MP analysis of a commercially available AAV control sample with predominantly (~95%) full/partial capsids, the team calculated expected values for the blended samples. The observed E:F ratios showed linearity with the expected values, with a coefficient of determination (R2) of ≥0.96. Because the full and partial species could be resolved, they also were analyzed independently, generating R2 values of ≥0.89 and ≥0.96, respectively.
In the webinar’s final case study, McManus described how Pharmaron used the SamuxMP system to optimize protocols for a chromatography process comprising one capture and two polishing steps. To evaluate the efficacy of the capture step, her team collected samples from the process eluate, analyzed them by MP, and compared the resulting mass-distribution profile and E:F calculation with the process chromatogram. MP effectively resolved the empty and full AAV capsids, indicating that full capsids accounted for 9.2% of virions in the eluate. Low-mass impurities were present on the mass-distribution plot, but they were resolved easily and made little impact on E:F calculation.
For the polishing steps, McManus’s team used the SamuxMP system to identify which elution fractions contained the highest percentages of full capsids. Three pooled fractions were collected from the first step. As expected, the mass distribution in the first fraction indicated the elution of low-mass contaminants, whereas the second and third fractions contained 16.6% and 6.1% full capsids, respectively. Those results were consistent with findings from the process chromatogram. Thus, material from the second fraction could be loaded for subsequent purification.
The final polishing step required MP analysis of four pooled fractions. Full-capsid percentages increased as the process continued, going from undetectable levels to 0.5%, 5.3%, and ultimately 59.9%. Thus, the final fraction could be leveraged to recover the greatest quantity of full capsids.
McManus reported that data generated by the SamuxMP system were consistent with results from process chromatograms gathered at each purification step. MP holds much promise as a simple, rapid, accurate, and cost-effective orthogonal method for AAV characterization.
Questions and Answers
What is the minimum vector concentration required by the SamuxMP instrument? AAV concentrations of 1010 vg/mL suffice, although the system operates best when titers are 1011 vg/mL.
Can MP be applied to analyze upstream AAV samples? MP is more useful for downstream development and optimization rather than for upstream activities because upstream samples (e.g., from bioreactor harvests) contain many impurities that limit MP’s effectiveness.
Can the SamuxMP instrument be used to study other viruses? It can be used to analyze virions with a diameter as large as 80 nm. (AAV particles have diameters of ~27 nm.)
What consumables are used during each measurement? Each run requires a glass coverslip with a “sample well cassette,” which is a silicone gasket containing a series of wells. Those consumables are available for purchase on the Refeyn website.
In Pharmaron’s experience, what have been the system’s most important advantages? Although the company has yet to integrate the instrument fully into its operations, the system holds much promise for reducing the amount of time, material, and capital required to determine E:F capsid ratios. AUC processes can require 400 μL of material with AAV concentrations of 1012 vg/mL. They also have limited throughput. The SamuxMP system would enable significantly higher throughput using much less product.
Find the full webinar online at www.bioprocessintl.com/category/webinars.
You May Also Like