Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
October 23, 2019
Sponsored by Eppendorf
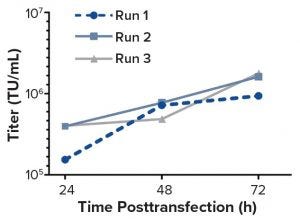
Figure 1: Functional titers (TU/mL) of the unconcentrated LV supernatant determined by using flow cytometric detection of GFP-positive cells
Although demand for lentiviral vectors (LVs) for cell and gene therapy is increasing, the standard two-dimensional culture systems used to produce LVs present significant disadvantages. Current bottlenecks in LV production are caused mainly by such disadvantages. Switching to use of bioreactors can eliminate those problems because bioreactors offer the benefits of process automation, tight regulation of production conditions, and reduced labor input. The study reported herein was carried out by the group of David Parsons at the University of Adelaide. It was one of the first attempts at transient LV production in a stirred-tank bioreactor with cells adherently grown on Fibra-Cel disks.
Just fill out the form below to read the full article now.
References
1 McCarron A, et al. Transient Lentiviral Vector Production in HEK293TCells Using the BioFlo 320 Control Station with a BioBLU 5p Single-Use Packed-Bed Vessel. Eppendorf Application Note 411, 2019.
2 Merten O-W, et al. Comparison of Different Bioreactor Systems for the Production of Higher Titer Retroviral Vectors. Biotechnol. Prog. 17(2), 2001: 326–335; doi:10.1021/bp000162z.
3 van der Loo JCM, et al. Scale-Up and Manufacturing of Clinical-Grade Self-Inactivating γ-Retroviral Vectors By Transient Transfection. Gene Therapy 19(3) 2012: 246–254; doi:10.1038/gt.2011.102.
4 Kohlstrom N., Sha M. Perfusion CHO Cell Culture in a BioBLU® 5p Single-Use Packed-Bed Vessel. Eppendorf Application Note 336. 2014.
5 Han X, Sha M. High-Density Vero Cell Perfusion Culture in BioBLU® 5p Single-Use Vessels. Eppendorf application note 359, 2017.
6 Holic N, et al. Influence of Mildly Acidic pH Conditions on the Production of Lentiviral and Retroviral Vectors. Hum. Gene Clin. Ther. Dev. 25(3) 2014: 178–185; doi:10.1089/humc.2014.027.
7 Xiaofeng H, Sha M. High-Density Vero Cell Perfusion Culture in BioBLU 5p Single-Use Vessels. Eppendorf application note 359, 2017.
David Solbach is scientific communications manager of bioprocess at Eppendorf AG, Rudolf Schulten-Str. 5, 52428 Jülich, Germany, 49-2461-980-168; [email protected].
You May Also Like