Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Sponsored by Thermo Fisher Scientific
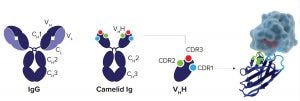
Figure 1: Regular IgG antibody compared with a Camelid single domain Ig antibody and VHH antibody fragment; the image on the far right shows VHH bound to its target protein.
The rapid and cost-effective production of conventional monoclonal antibodies (MAbs) for clinical trials and commercial supply has contributed toward their wide adoption. Production processes have become more efficient because common purification processes are being used across structurally similar MAbs during key steps of process development and manufacturing. Such successful platform approaches can remove unwanted impurities and are stable across processing conditions, irrespective of the MAb being purified. In addition, they are readily available at the required volume to support large-scale current good manufacturing practice (CGMP) production and can be validated easily. Platform approaches also minimize both the duration and number of steps in downstream processing operations, which contributes significantly to maximizing efficient batch production.
This article chronicles the development of CaptureSelect technology, a suite of affinity ligands based on single-domain antibody fragments. Just fill out the form below to read it now.
Reference
1 Scanlan C, et al. Downstream Purification and Formulation of Fab Antibody Fragments. BioPharm Int. 27(1) 2014.
Corresponding author Frank Detmers is director of ligand application at Thermo Fisher Scientific. Jan Griesbach is manager of development recovery and DSP at F. Hoffmann, La Roche. Kevin Sleijpen is a scientist at Thermo Fisher Scientific. Jaco Hazenoot is a scientist at Thermo Fisher Scientific. Pim Hermans is director of ligand discovery at Thermo Fisher Scientific, J.H. Oortweg 21, 2333.
You May Also Like