Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
August 15, 2014
 Given the current drive to reduce the cost of manufacturing for biological therapeutics, it is incumbent upon chromatographers and process engineers to streamline the manufacturing process wherever possible. As a result of this need, the demands on downstream unit operations have increased to the point where a single processing step is expected to accomplish a multitude of purification objectives.
Given the current drive to reduce the cost of manufacturing for biological therapeutics, it is incumbent upon chromatographers and process engineers to streamline the manufacturing process wherever possible. As a result of this need, the demands on downstream unit operations have increased to the point where a single processing step is expected to accomplish a multitude of purification objectives.
Introduction
Anion exchange chromatography is a widely used technique for protein capture or impurity removal. Typically, anion exchange resins with quaternary amine or DEAE ligands are used. However, these more conventional resins have the disadvantage of reduced capacity for proteins in relatively high salt concentrations associated with post-protein A purification monoclonal antibodies (mAbs) or undiluted biological feedstock. In order to use a DEAE or quaternary amine resin at these process stages, the column load material must be diluted to adjust its conductivity to approximately 5 mS/cm.
TOYOPEARL NH2 -750F, a salt tolerant anion exchange resin for process scale applications, was recently introduced by Tosoh Corporation. This resin is based on the TOYOPEARL HW-75F size exclusion resin functionalized with primary amine groups. This allows the TOYOPEARL NH2 -750F resin to maintain its capacity at conductivities up to 15 mS/cm.
Table 1 lists the properties of the TOYOPEARL NH2 -750F resin.
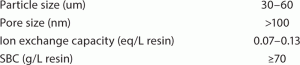
TABLE 1: Properties of TOYOPEARL NH2 -750F
This new resin uses the same polymethacrylate backbone as all other TOYOPEARL chromatography resins. Thus, it has similar pressure-flow and chemical stability characteristics. TOYOPEARL NH2 -750F resin is ideal for the intermediate (post-protein A) purification of mAbs where aggregates and other impurities are removed from the target of interest without having to dilute or buffer exchange the product prior to loading.
The data presented here demonstrate the capabilities of TOYOPEARL NH2 -750F to remove dimer and higher order aggregates from the monomer of a protein A purified IgG1 monoclonal antibody.
Experimental Conditions/Results
Figure 1 is a size exclusion chromatography analysis of an aliquot of the protein A purified IgG1 mAb on a TSKgel G3000SWXL column. This sample is representative of the material that will be loaded onto the TOYOPEARL NH2 -750F column. In this figure, the presence of monomer, dimer, and higher order aggregates can be seen. The presence of a peak at greater than ten (10) minutes retention time is due to residual citrate from the protein A elution step.
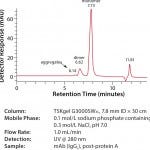
FIGURE 1: SEC analysis of mAb prior to aggregate removal by TOYOPEARL NH2 -750F
For the dimer/aggregate removal experiment, a 5 mm ID 5 cm column was packed with TOYOPEARL NH2 -750F resin. The column was equilibrated with 20 mmol/L Tris-HCl, pH 8.0 (mobile phase A) for 5 CV. A 0.5 g/L sample of protein A purified mAb (IgG1) was then loaded onto the column, and the column was washed with mobile phase A.
A 60 minute linear gradient from 0 to 100% mobile phase B (mobile phase A + 1.0 mol/L NaCl) was used to separate the mAb monomer (peak 1) from mAb dimer and aggregates (peak 2). The individual peaks were collected as fractions 1 and 2 (Figure 2). After elution, the column was sanitized using 0.5 mol/L NaOH.
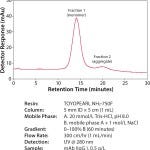
FIGURE 2: Purification of mAb on TOYOPEARL NH2 -750F column.
The purified mAb monomer (fraction 1) and the mAb aggregate (fraction 2) peaks were analyzed by size exclusion chromatography using a TSKgel G3000SW
XL column to verify the separation of dimer and aggregates from the mAb monomer.
There was no dimer or higher order aggregate present in the mAb monomer peak, as can be seen in Figure 3. Analysis of the aggregate peak in Figure 4 does indicate that a small amount of mAb monomer was co-eluted with the dimer and aggregates in this experiment. The higher order aggregates were visible in Figure 1 but were not visible in Figure 4 . This is possibly due to either sample dilution or that the aggregates were removed in the sanitization step.
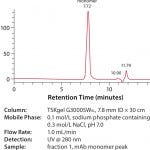
FIGURE 3: SEC analysis of purified mAb monomer from TOYOPEARL NH2 -750F (fraction 1)
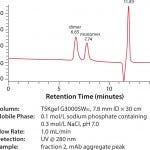
FIGURE 4: SEC analysis of mAb aggregate peak from TOYOPEARL NH2 -750F (fraction 2)
Conclusions
TOYOPEARL NH2 -750F is an effective anion exchange resin for the removal of dimer and higher order aggregates from mAb monomer in a protein A purified antibody without the need for sample dilution or buffer exchange. Though some monomer was co-eluted with the dimer and aggregates in this experiment, further method development may reduce the amount of carryover and increase product yield in the monomer peak.
Tosoh Bioscience, TSKgel and TOYOPEARL are registered trademarks of Tosoh Corporation.
R. Christopher Manzari is the process chromatography marketing manager at Tosoh Bioscience LLC, 3604 Horizon Drive, Suite 100, King of Prussia, PA 19406; 1-484-805-1219; [email protected]; www.tosohbioscience.com
You May Also Like