Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
June 21, 2022
Sponsored by BIA Separations
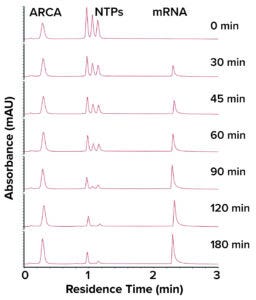
Figure 1: Chromatogram from analysis of an IVT reaction using a CIMac PrimaS monolith chromatography column.
In vitro transcription (IVT) is a critical step in messenger RNA (mRNA) production. In a March 2022 webinar, Rok Sekirnik (head of process development for mRNA and plasmid DNA (pDNA) applications at BIA Separations, part of Sartorius) explained that optimizing concentrations of reagents used during IVT helps to maximize the amount of mRNA that is produced. Drug developers have great need for analytical methods that can measure IVT in real time. Sekirnik showed how CIMac PrimaS monolith chromatography columns enable at-line analysis of IVT, including concentration monitoring of nucleoside triphosphates (NTPs), capping reagents, and plasmids.
The Presentation
Sekirnik noted that a 4,000-nucleotide mRNA molecule such as that featured in mRNA vaccines against SARS-CoV-2 is ~1.3 MDa — approximately 10× larger than a standard IgG antibody. Messenger RNA also carries a negative charge and becomes hydrophobic when exposed to high salt concentrations. To enable protein translation, it must have a 5′ cap and polyadenylated (poly-A) tail. Poly-A tails can be encoded into the DNA template used for transcription or added enzymatically after IVT. Similarly, 5′ caps can be added during or after IVT.
Considering such properties, drug developers need analytical methods that can measure mRNA yield and capping efficiency in real time. High-performance liquid chromatography (HPLC) is helpful for such evaluations, but analysts must select a separation medium that provides a suitable binding chemistry, a sufficiently large surface area for mRNA, and capability for rapid analyses. In bead-based media containing porous particles, diffusion limits mass transfer, requiring long analysis times and limiting potential for at-line assessment. Beads also generate countercurrent flow, producing shear stress that diminishes recovery.
CIMac PrimaS monolith columns rely on convective mass transport through a polymethacrylate stationary phase. Separation is based on anion-exchange and hydrogen-bond multimodal interactions. Laminar flow through the column minimizes shear stress. Compared with porous particles, the microchannels provide much more surface area for mRNA binding. Because the channels lack dead-end pores, their binding capacity and resolution remain the same across flow rates.
That feature enables analysts to process samples quickly enough to evaluate IVT in real time without diminishing resolution. Sekirnik noted that one assay can separate and quantify mRNA, pDNA, capping reagents, and distinct NTPs (Figure 1). Results are generated in less than three minutes.
Such capabilities enable monitoring and control of mRNA production kinetics, including 5′ capping efficiency. Sekirnik explained that if an exonuclease that selectively degrades uncapped mRNA is used, then a sample can be analyzed for mRNA concentration, treated enzymatically, and analyzed again to quantify the undigested molecules. Comparing concentration values from before and after treatment will reveal an IVT reaction’s capping efficiency. Analysts can adjust feeding regimens and reagent concentrations accordingly to optimize mRNA capping and yield.
Sekirnik demonstrated that possibility using data from a fed-batch IVT process. Sartorius initiated IVT with a 1:4 ratio of guanosine triphosphate (GTP) to antireverse cap analog (ARCA). An hour into the process, capping efficiency was high (80%), but yield was low. The team then used a feeding strategy to adjust NTP levels throughout the reaction while maintaining the GTP:ARCA ratio. Such changes increased yield sixfold (to
11.7 mg/mL) without diminishing capping efficiency (80%) or product quality.
Using such a workflow, analysts can obtain information rapidly about both capping efficiency and yield. Sartorius PATfix software includes preloaded analytical sequences and tools to facilitate process monitoring. The company also offers monolith columns with other binding chemistries to provide multiple tools for mRNA manufacturing and process analytics.
Questions and Answers
Can CIMac PrimaS columns be used in HPLC systems with quaternary pumps? CIMac analytical columns can be used with HPLC systems; quaternary pumps are preferred.
Can the columns characterize mRNA polyadenylation? CIMac Oligo dT columns can be used to quantify polyadenylated mRNA content.
The recorded webcast is now available to view on-demand: Watch Now.
You May Also Like