Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
June 18, 2021
Sponsored by Thermo Fisher Scientific
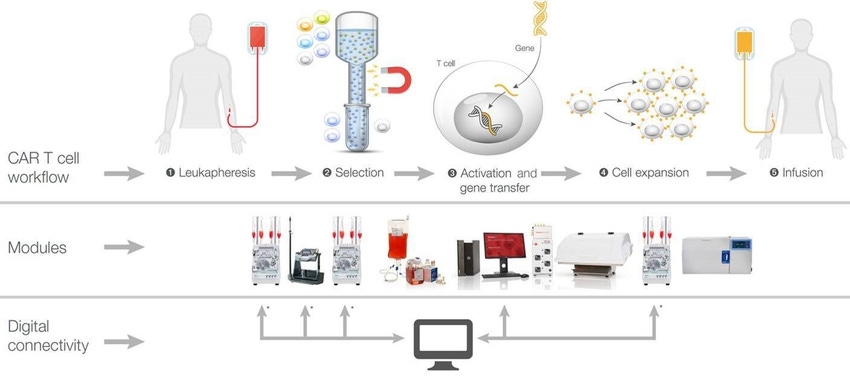
Cell-based chimeric antigen receptor (CAR) T cell therapies have rapidly advanced in recent years, with a variety of targets in clinical research and several FDA approved products already on the market. There has been tremendous effort to make CAR T cells more effective, safe, and persistent when treating patients. On the manufacturing side, however, errors, lot-to-lot variation, and contamination can be associated with open processes and manual handling of CAR T cells.
Cell isolation, gene editing, expansion, and cryopreservation are complex steps in a typical autologous CAR T cell manufacturing process. Integrating this complicated multistep workflow into a closed, modular, benchtop system can facilitate a transition from the laboratory to clinical manufacturing while improving consistency, purity, and safety. Thermo Fisher Scientific presents a digitally compatible, GMP-compliant, semiautomated manufacturing platform, which when used with Gibco™ CTS™ reagents, protocols, and analytics can result in consistent, efficacious CAR T cell production. The system leverages the DeltaV™ Distributed Control System from Emerson to control and manage the instruments in the workflow.
You May Also Like