Insider News
Latest Issue
Current Issue
Advanced Saccharide Solutions in Biopharmaceutical Development: Paving the Way for Product Stability and Environmental Sustainability
BioProcess Insider Daily - Biotech Week Boston
Read MoreAndrew Mears, CEO of Lead Candidate, highlighted a dichotomy in the industry, where some sectors are downsizing while others are experiencing rapid growth during a video interview at Biotech Week Boston (BWB).

Experts call for better analytics, customization, R&D, and strategic investments as cell and gene therapies struggle with funding challenges and complex manufacturing needs.

Aurigene’s chief commercial officer says his firm and other Indian CDMOs are “a very viable alternative” if major Chinese manufacturers are removed from the US biopharma supply chain.
Cell therapies will play an increasingly important position in AstraZeneca’s broad oncology pipeline, an SVP told delegates at Biotech Week Boston (BWB) last month.
Latest eBooks
See AllSpecial Reports
The Cydem VT system combines multiple trusted Beckman Coulter Life Sciences technologies into one advanced platform that empowers lab managers with a far more effective, higher-throughput method for top clone screening in Cell Line Development—offering increased confidence that promising clones don’t get overlooked in the process of consistently selecting the best. Key features: Register or Login and hit Download Now to get the full Special Report.
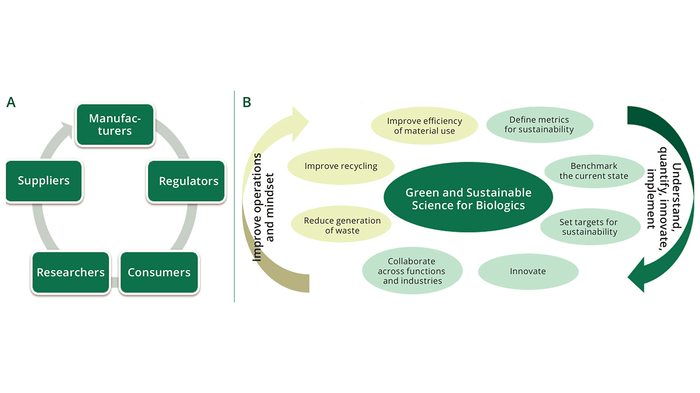
The journey from preclinical development to commercialization of a new biopharmaceutical is long and challenging. The entire process requires persistence, adaptability, and strategic foresight. Only with a well-coordinated effort across disciplines and business functions can a new biologic drug successfully advance from laboratory to market. Partnering with a CDMO can be invaluable in navigating such challenges to provide flexibility, enabling drug sponsors to focus on their core competencies while relying on their partners to handle the intricacies of manufacturing and regulatory compliance. Such partnerships can reduce risks significantly and help ensure that a new biologic reaches the market as efficiently as possible. Learn key considerations for the journey to commercialization. Register or Login and hit Download Now to get the full Special Report.
Ask the Expert Webcasts
This webcast features: Janet Lee, director, cell line development, Samsung Biologics. The increasing complexity of biopharmaceutical molecules is driving innovation in cell line development (CLD) to enhance productivity and functionality. To address these evolving demands, Samsung Biologics has developed two advanced platforms: S-CHOice® and S-AfuCHO™. The S-CHOice® platform, powered by transposase technology, tackles common CLD challenges such as low protein expression, multi-chain molecule complexity, and clonal instability. It ensures efficient and scalable biologics production while meeting stringent timelines and reducing product impurities. The S-AfuCHO™ platform features an afucosylated CHO cell line engineered through FUT8 knockout technology. By removing core fucose from the antibody’s Fc region, it enhances binding affinity to FcγIIIa receptors, significantly improving antibody-dependent cellular cytotoxicity (ADCC) functionality. Join this webinar to learn how these platforms optimize CLD pe...
This webcast features: Joe Payne, president & COO & Jeff Wolf, founder & CEO, Scorpius BioManufacturing. Too many promising biologic candidates are stalled without critical early-stage funding. Grant funding often falls short of the capital needed for pre-clinical and phase 1 manufacturing, but dilutive capital is hard to secure without positive early-stage data. In this webinar, learn about a hybrid model for capital-efficient access to cGMP manufacturing, process development, and analytical services for early-stage biotech companies through in-kind equity investment. Hear from Scorpius’ president and founder about why this US biologics CDMO was inspired to develop an innovative funding model to support innovative biotech companies. Thank you for your interest in this webcast. You may submit your request to view the webcast, and you will receive a confirmation email with log-in details.