Measurement of Particulates in Single-Use Systems for Cell and Gene Therapies Manufacturing — Part 1: Misapplication of USP <788>

Production of SUS equipment in a cleanroom.
Particulates are mobile, undissolved particles other than gas bubbles that are unintentionally present in an injectable drug product. Patient safety can be compromised by the amounts and types of particulates present in a drug product (1). They differ in nature (e.g., metal, glass, dust, fiber, rubber, polymer, mold, and degradant precipitates) and can be divided into three categories: intrinsic, inherent, and extrinsic particulates.
Intrinsic (native) particles are derived from operation of a manufacturing process or its equipment, a product formulation, or a container system.
Inherent (formulation) particles are innate product characteristics (e.g., adjuvants in vaccines and lipid nanoparticles (LNPs)) used as drug delivery vehicles).
Extrinsic (foreign) particles originate from a manufacturing environment and are foreign to a manufacturing process.
Regulatory requirements for final drug products and biopharmaceutical manufacturing processes are clear. They spell out the expectations for equipment, surfaces, and all particulates that might be contained on the surfaces of processing equipment. The US FDA’s current good manufacturing practice (CGMP), 21 CFR 211.65 states:
Equipment shall be constructed so that surfaces that contact components, in-process materials, or drug products shall not be reactive, additive, or absorptive so as to alter the safety, identity, strength, quality, or purity of the drug product beyond the official or other established requirements. (2)
The US FDA CGMP for biologicals, 21 CFR 600.11, similarly states:
All surfaces that come in contact with products shall be clean and free of surface solids, leachable contaminants, and other materials that will hasten the deterioration of the product or otherwise render it less suitable for the intended use. (3)
The ICH Q7 Good Manufacturing Practice for Active Pharmaceutical Ingredients guideline states:
Equipment should be constructed so that surfaces that contact raw materials, intermediates, or APIs do not alter the quality of the intermediates and APIs beyond the official or other established specifications. (4)
Multiuse process tanks are usually rinsed with water for injection (WFI) as the last cleaning step or before they are charged with process fluids. Rinsing with WFI usually reduces the surface particulate load significantly, especially after long storage periods.
A clear benefit of SUS has surely been the enablement of closed-system biopharmaceutical production and processing with no clean-in-place (CIP) or steam/sterilization-in-place (SIP) steps required. That has greatly reduced the risks from environmental, water, and batch-to-batch contaminants in drug products. However, risks from particulates can increase when SUS are used in bioprocessing steps because particulates usually have not been rinsed from SUS materials during their manufacture. As the BPSA paper on particulates indicates (5), SUS manufacturers have addressed such particulate risks by performing a large majority of manufacturing and assembly processes in ISO-classified clean rooms. SUS manufacturing requirements have improved steadily. It now is common to manufacture SUS in ISO 7 clean rooms with continuous monitoring and product testing.
Both multiuse and single-use biopharmaceutical production processes control particulate risks with purification processes that implement 0.2 µm filtration at critical points within a process and for final drug product filtration. However, appropriate validated procedures need to be implemented when filters are used. As defined in the US FDA 21 CFR 210 on CGMPs:
Nonfiber releasing filter means any filter, which after appropriate pretreatment such as washing or flushing, will not release fibers into the component or drug product that is being filtered. (6)

Visual inspection of SUS process equipment for visible particles >100 μm.
Drug product manufacturing using sterile 0.2 µm filters works well to remove particulates, including those that result from a manufacturing process (e.g., storage vessels and peristaltic pump tubing). Thus, the areas of concern are reduced to final drug product fill–finish activities and those therapies that cannot be filtered (e.g., vaccines). Final drug products are monitored for visible particulates (>100 µm) by 100% visual inspection of vials and syringes per USP <1> Injections (7) and USP <790> Visible Particulates in Injections (8), whereas subvisible particulates (<100 µm) are monitored by light obscuration and membrane microscopy, per USP <788> Particulate Matter in Injections (9).
However, monitoring and control of particulates in cell therapy manufacturing present new challenges. Cell therapies are manufactured by complex multistep processes that contain many potential sources for introduction of particulate matter (10). Cell therapies are suspensions of living cells (typically between 10 and 30 µm), so application of 0.2 µm sterile filters will remove the active ingredient (the cells themselves). Thus, there is no opportunity to take advantage of filtration processes to remove unwanted particulates. Another challenge is that cell suspensions often are not fully transparent, which limits the effectiveness of visual inspections. Also, light obscuration for subvisible particle monitoring cannot differentiate cells from extrinsic particulates. Particulate contamination in CGT products can present significant risks to patient safety because many of those products are administered through intravenous injection. It is therefore especially crucial to limit particulates from all possible sources, including particulates from SUS process equipment used to manufacture cell therapies.
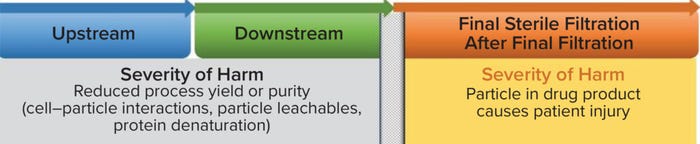
Figure 1: Probability of occurrence (binary risk) in biopharmaceutical manufacturing.
Applying USP <788> To Measure Particulates in SUS
No standardized test methods exist for measuring particulate matter in SUS. The absence is compensated for by “applying” the USP <788> test method for injectable drug products to SUS. However, that forced application, although convenient, will not detect both subvisible and visible particulates in SUS, and it thus will not give a realistic measure of particulates.
USP <788> describes two test methods for measurement of particulate matter in parenteral drug products. In “Method 1: The Light Obscuration (LO) Particle Count Test,” drug product flows through a sensor comprising a flow cell, a laser light beam, and a photodiode detector. A particle is counted and sized when it absorbs and/or scatters the light beam, which the photodetector registers as an attenuated signal. Through calibration of the signal attenuation obtained from spherical reference particles of known diameter, the signal from an unknown particle is converted into an effective spherical diameter. The LO technique is quick, automated, and robust, with a nearly 40-year history of use in the measurement of particles in parenteral drug products.
In an alternative test method described in USP <788> as “Method 2: Microscopic Particle Count Test,” a drug product is passed through a membrane filter, and under specific illumination conditions, an operator compares the particles on the membrane filter with circular reticules 10 µm and 25 µm in diameter. This technique is essentially a visual inspection of particles on the surface of a membrane filter, aided by a microscope. An operator visually compares a particle with a circular reticule and decides whether the particle is larger or smaller than the reticule’s diameter. An advantage of membrane microscopy is that particulates are isolated from a solution and, if large enough, can be characterized further after filtration and counting (e.g., by FTIR/Raman microscopy or SEM-EDX). The nominal pore size of the membrane filter sets the lower limit on particle size trapped upon filtration. The upper particle size limit is limited only by the diameter of the membrane filter (usually 25 or 47 mm). Thus, particles in the visual size range (>100 µm) are detected.
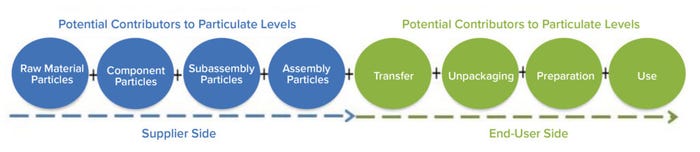
Figure 2: Potential contributors to particulate levels.
Discussion: Unsuitability of USP <788> for Single-Use Systems
The intended use of USP <788> is for parenteral drug products — not SUS: The titles of USP <788>, <789>, and <787> (Particulate Matter in Injections, Particulate Matter in Ophthalmic Solutions, and Subvisible Particulate Matter in Therapeutic Protein Injections, respectively) make it clear that those guidance chapters are applicable to particulate matter in parenteral drug products. The chapters were not intended for, nor do they address measurement of particulates in single-use systems.
There is no description of particulate extraction procedures for SUS in USP <788>: Liquid extraction of particulates from SUS surfaces is arguably the most critical step for reliable characterization and/or monitoring of a particulate population present in a system. The effectiveness of an extraction method is influenced by the properties of a single-use system as well as by the wetting and suspension properties of a test liquid, temperature, energy input (rinsing, agitation), and the length of time the surfaces of a test article are exposed to that test liquid. However, USP <788> provides no guidance for or procedures applicable to extracting particulate matter from single-use systems. This lack of a standardized extraction procedure potentially results in test samples that do not represent the actual particulate risk in CGT products.
The LO Technique in USP <788> Has Limitations When Applied to SUS: According to USP <788>, LO is one of the most common analytical techniques in pharmaceutical quality control laboratories. LO has been a mandatory standard method for quality control and release of parenteral drug products for decades and is accepted by regulators as the de facto standard for subvisible particle analysis. Through its widespread use and acceptance, most pharmaceutical quality control (QC) professionals are familiar with LO, and the required instrumentation is readily available in most CGMP laboratories.
As indicated in the guidance given in USP <1788> and <1788.1>, the LO technique is limited to measurement of particles <300 µm depending upon instrument configuration (11). Because LO is an indirect measurement of particle size, for fibers longer than the illumination zone, it will report a diameter closer to the diameter of the fiber than to the length of the fiber. Also, for particles with low contrast against the liquid medium, the particle size reported will be smaller than the actual diameter or might not be detected at all. LO not only will detect solid particles, but it also will detect liquid droplets and air bubbles and cannot differentiate between them. Although some LO instrument manufacturers claim detection of particles up to 600 µm, according to Barber (12), the upper limit on particle size measured with LO depends not only on sensor dimensions, but also upon particle size, density, and morphology. As particle size, aspect ratio (ratio of length to width), and density increase, sampling errors increase because larger particles might not be drawn up reliably to flow through the sensor. Although LO is a well-accepted technique for the measurement of subvisible particles (<100 µm), LO will therefore not detect all potential particles in the visible size range (>100 µm) reliably.
Membrane Microscopy: As indicated in the guidance provided in USP <1788> and <1788.2>, manual membrane microscopy is labor intensive and fatiguing, especially when many particles are present on a membrane filter. Although manual membrane microscopy is a well-accepted technique for detection and size classification of particles in both subvisible and visible size ranges, the reliance on manual counting and sizing large numbers of particulates makes it subject to human error. A more efficient technique with a higher degree of automation is needed.
 Reporting and Acceptance Criteria in USP <788> Are Not Appropriate for SUS: To assess risk to a final drug product effectively, particulate counts are needed per component/assembly or per surface area in contact with the fluid together with total fill volume or total fluid-path contact area. USP <788> requires that all detected particle counts are reported per milliliter of drug product for large-volume parenteral products. In our opinion, this type of reporting is inappropriate for SUS and is not fit for the intended use of the data. The nonstandardized force-fit of USP <788> to SUS allows for “creative” adaptations. For example, increasing the volume of liquid used to extract particles from the surfaces of single-use systems will lead to a lower reported particle count if those particles are reported per milliliter of liquid applied in the extraction. Calculation in this way simply “dilutes” the particles and does not represent the particle load of that SUS component. A suggested approach is to add a particulate requirement similar to that in USP <161> Medical Devices — Bacterial Endotoxin and Pyrogen Tests. That method uses procedures from USP <85> Bacterial Endotoxins for the assays but reports the limits per medical device.
Reporting and Acceptance Criteria in USP <788> Are Not Appropriate for SUS: To assess risk to a final drug product effectively, particulate counts are needed per component/assembly or per surface area in contact with the fluid together with total fill volume or total fluid-path contact area. USP <788> requires that all detected particle counts are reported per milliliter of drug product for large-volume parenteral products. In our opinion, this type of reporting is inappropriate for SUS and is not fit for the intended use of the data. The nonstandardized force-fit of USP <788> to SUS allows for “creative” adaptations. For example, increasing the volume of liquid used to extract particles from the surfaces of single-use systems will lead to a lower reported particle count if those particles are reported per milliliter of liquid applied in the extraction. Calculation in this way simply “dilutes” the particles and does not represent the particle load of that SUS component. A suggested approach is to add a particulate requirement similar to that in USP <161> Medical Devices — Bacterial Endotoxin and Pyrogen Tests. That method uses procedures from USP <85> Bacterial Endotoxins for the assays but reports the limits per medical device.
Furthermore, the acceptance criteria in USP <788> are intended for, and apply to, final drug product and are thus inadequate for particulate monitoring in SUS. This absence of applicable method and/or material specifications is scientifically and regulatorily problematic. Although these assessments are generally performed by component suppliers, the compendial standards are likely to be incorrectly applied to SUS commonly used during cell therapy qualification and manufacturing activities. The BPSA paper on particulates clearly discusses the risks from both the manufacture and use of SUS.
Applying Scientific Best Practice and Evidence-Based Testing
In summary, light obscuration testing citing USP <788> often is applied to SUS as a checkbox activity to satisfy customers and regulators instead of following scientific best practice and risk- and evidence-based testing strategies. USP <788> and by extension, associated and harmonized guidance chapters such as USP <787>, <788>, <789>, and <1788> and European Pharmacopoeia 2.09.19 clearly have significant shortcomings when applied to control of particulates in SUS. Although analytical technology such as light obscuration or membrane microscopy can be used to measure particulates extracted from SUS, USP <788> and the chapters mentioned above never were intended for — nor are they applicable to — single-use systems.
Moreover, the lack of a suitable extraction procedure, as well as inadequate reporting and acceptance criteria, may lead to misrepresentations of a particle load and thus severely misrepresent the associated risks to patient safety.
Therefore, we believe that a standardized method that generates SUS particulate data that is fit for purpose (such as automated microscopy) is desperately needed. In subsequent papers we will address best practices for the measurement of particulates in single-use systems, focusing on development and validation of an extraction procedure, best methods for detection of both subvisible and visible particles, and formats for exchanging essential particulates data between suppliers and end users. Such discussions coincide with a recently published standard practice on extraction of particulates from single-use systems (ASTM E3230-20), multiple task groups in the ASTM E55 committee on Biopharmaceutical Processing are drafting standards to address the gaps in measurement and reporting of particulates in single-use systems.
Acknowledgment
The authors thank Maureen Eustis, technical services coordinator at The BioProcess Institute, for her assistance in preparing and reviewing this manuscript.
References
1 Bukofzer S, et al. Industry Perspective on the Medical Risk of Visible Particles in Injectable Drug Products. PDA J. Pharm. Sci. Tech. 69(1) 2015; 123–139; https://doi.org/10.5731/pdajpst.2015.01037.
2 21 CFR 211.65: Equipment Construction. US Code of Federal Regulations. US Food and Drug Administration: Rockville, MD, 2023; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=211.65.
3 21 CFR 600.11: Physical Establishment, Equipment, Animals, and Care. US Code of Federal Regulations. US Food and Drug Administration: Rockville, MD, 2023; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=600.11.
4 ICH Q7: Good Manufacturing Practice for Active Pharmaceutical Ingredients. US Fed. Reg. 66(186) 2000: 49028–49029; https://database.ich.org/sites/default/files/Q7%20Guideline.pdf.
5 Bio-Process Systems Alliance. BPSA 2020 Recommendations for Testing, Evaluation, and Control of Particulates from Single-Use Process Equipment; https://www.scribd.com/document/486229412/BPSA-2020-Recommendations-for-Testing-Evaluation-and-Control-of-Particulates-from-Single-Use-Process-Equipment.
6 21 CFR Part 210.3.b.6: Current Good Manufacturing Practice in Manufacturing, Processing, Packaging, or Holding of Drugs; General. US Code of Federal Regulations. US Food and Drug Administration: Rockville, MD, 2023; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=210.3.
7 USP–NF <1>. Injections and Implanted Drug Products (Parenterals) — Product Quality Tests. United States Pharmacopeia: Rockville, MD, 2016; https://www.usp.org/sites/default/files/usp/document/harmonization/gen-method/q08_pf_31_1_2005.pdf.
8 USP–NF <790>. Visible Particulates in Injections. United States Pharmacopeia: Rockville, MD, 2014; https://doi.org/10.31003/USPNF_M7197_01_01.
9 USP–NF <788>. Particulate Matter in Injections. United States Pharmacopeia: Rockville, MD, 2012; https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisionGeneralChapter788.pdf.
10 Clarke D, et al. Managing Particulates in Cell Therapy: Guidance for Best Practice. Cytotherapy 18(9) 2016; 1063–1076; https://doi.org/10.1016/j.jcyt.2016.05.011.
11 USP–NF <1788>. Methods for the Determination of Subvisible Particulate Matter. United States Pharmacopeia: Rockville, MD, 2012; https://doi.org/10.31003/USPNF_M3023_02_01.
12 Barber TA. Control of Particulate Contamination in Healthcare Manufacturing. CRC Press, Boca Raton, FL, 1999; https://doi.org/10.1201/9780429246692.
Corresponding author James Dean Vogel, PE, is the director of the The BioProcess Institute, 76 Dry Bridge Road, Unit H-3, North Kingstown, RI 02852; 401-294-9000; [email protected]. Andreas Hohenleutner is head of characterization and device services at Solvias, Joseph St. Laurent is head of industry relations and is a regulatory expert at Solvias, and Klaus Wormuth is a principal scientist at Sartorius.
You May Also Like





