Driving Sustainability in Biologics Process Development: Innovative Strategies and Technologies
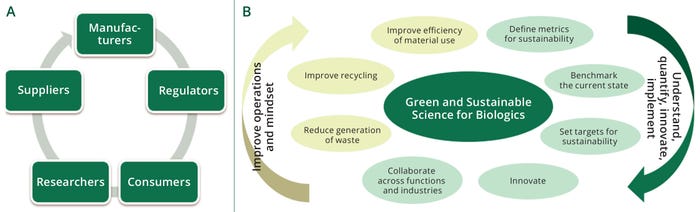
Figure 1: (a) Overview of innovation network/collaboration required for sustainability; (b) a multifaceted approach to achieving sustainability.
Development and implementation of technologies to support new biopharmaceutical products and processes require companies to take a holistic and collaborative approach to achieve their technical, financial, and sustainability goals (Figure 1). Effectively, a sustainability ecosystem needs to be created toward the common industry goal of innovating, improving, and implementing disruptive technologies that can improve sustainability significantly.
We can draw some inspiration from the origins of the biotechnology community, which came about through a number of innovations. Once daunting tasks, including how to create lifesaving therapies using Chinese hamster ovary (CHO) cells, have become familiar technologies exemplified by products such as Herceptin (trastuzumab) and Keytruda (pembrolizumab) (1, 2). Our most audacious current goals also can be achieved through a systematic approach that begins with problem definition followed by application of creativity, strong fundamental science, engineering, and tenacity. Eventually, collaboration toward a shared objective will create, optimize, and apply the necessary technology, and the problem will be solved. Consider the example of mRNA vaccines during the COVID-19 pandemic (3). Society — including the biotechnology community — faces many significant challenges, including climate change. Imagine what could be achieved if we as a community applied a concerted effort such as that brought to bear for product and process development to the question of sustainability as well.
Many companies and consortia are working to establish targets for sustainability and create new technologies accordingly. Given the complexity of the endeavor, multiplicity of stakeholders involved, and large number of decision-makers engaged in biopharmaceutical innovation, supply chain, process development, and manufacturing, it is essential to establish and sustain a community to support the realization of environmental sustainability (4). Some efforts are underway at leading organizations such as the American Chemical Society’s Green Chemistry Institute Pharmaceutical Roundtable (ACS-GCI-PR), the Biopharma Focus Group, and the sustainability workstream of the US National Institute for Innovation in Manufacturing Biologics (NIIMBL), as well as companies such as Novartis AG, Cytiva (Danaher Corporation), Sartorius AG, and Merck & Co., Inc. of Rahway, NJ, USA (MSD).
For scientists and engineers, the climate and environmental crisis presents both an opportunity and an obligation. Bioprocessing is a resource-intensive enterprise with a process mass intensity (PMI) index over 10× higher than that of small-molecule active pharmaceutical ingredients (APIs), and water use accounts for more than 90% of PMI (5, 6). Reducing the environmental footprint of bioprocessing while producing therapeutic biologics as rapidly as possible will save lives both directly through medical intervention and indirectly through benefitting the environment. The bioprocessing footprint extends from laboratory (discovery, bioprocess, and pharmaceutical science) activities through pilot plants to clinical and commercial manufacturing. One promising solution is process intensification for upstream production, including intensified fed-batch cultures at extremely high cell densities and product concentrations. Opportunities for improvements in downstream processing include operating high-capacity chromatography resins at higher concentrations, reducing pool volumes and filter area (through increased membrane load volumes), and applying single-pass tangential-flow filtration (SPTFF) to in-process concentration operations. New filter and membrane technologies are improving membrane utilization with increased filter loading based on enhanced scientific understanding of fouling mechanisms.
Translation of the abstract concepts of climate change (including decarbonization) into practical terms is addressed herein through specific examples in which new technologies and processes have been applied to increase sustainability.
Where To Begin
Currently, a gap exists in translating climate impacts — e.g., measured in metric tons of CO2 based on the US Department of Energy’s 2020 decarbonization road map — into financial penalties or incentives that could drive innovation and investments (7). According to that report, industry represents 30% of the primary energy-related CO2 emissions, or 1.36 billion metric tons of CO2, in the United States. However, the impacts of those CO2 emissions are unclear — and so is the value of investing in technology to reduce those emissions and other detrimental environmental impacts (e.g., consumption of water and nonrenewable energy).
Here are some key questions to consider when working to translate the abstract concept of sustainability into a business process:
• How do we consider environmental impact in making financial and business decisions about process development and new technologies?
• What is the influence of research and development (R&D) on the environmental impacts of biomanufacturing (considering that results can have a multiyear lag)?
• What can we do as biopharmaceutical scientists and engineers to reduce the environmental footprint of our activities?
• What is the multiplicative effect of translating sustainable approaches to research into manufacturing platforms?
Establishing specific, quantifiable targets and goals initiates the process and helps to define the endpoint. Varied and numerous are the opportunities, paths, and approaches that the industry could take to achieve its goals. That makes it difficult to connect the abstract to any specific project goals.
Following one of those paths, MSD has established specific corporate goals related to climate, greenhouse gas (GHG)–emission science-based targets (SBTs), renewable energy, water use, and waste (Table 1). Once such goals have been established, it is incumbent on each business unit and project team to identify opportunities for sustainability as part of the business process for developing technologies and products. The ultimate goal of green and sustainable science (GSS) at MSD is for the company to become recognized as the industry leader in designing sustainable commercial processes regardless of drug modality (8).

Table 1: Overview of sustainability goals at Merck & Co., Inc. of Rahway, NJ, USA (MSD).
* We define purchased electricity as that sourced from external suppliers, including renewable electricity generated and used onsite,
for which we either retained the renewable attributes or have obtained renewable attributes through contract.
As summarized in Figure 2, the influence of manufacturing locations and supply-chain requirements — combined with the types of technology applied (single-use production) — has been evaluated in detail by others (9). One possibility is colocation of drug-substance (DS) and drug-product (DP) manufacturing at a single site, which potentially eliminates the need for energy-intensive, controlled-temperature storage and transportation of DS.
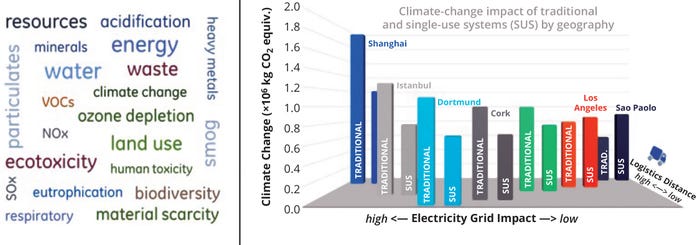
Figure 2: Comprehensive assessment of environmental impacts of traditional and single-use manufacturing networks specific to climate change (adapted from 9).
A Proposed Approach
To consider sustainability in bioprocess development, we suggest mapping all unit operations and associated raw materials and facility requirements relative to their effects on climate change, freshwater use, GHG emissions, human toxicity (both cancer and noncancer effects), photochemical ozone formation, and resource depletion (10). Once the initial mapping is complete, then you can rank order the most valuable opportunities. For example, the seed train and production bioreactor are unit stages that account for the greatest burden because they have the longest duration (18 and 14 days, respectively) and thus require a greater allocation of cleanroom energy. Hence, improving productivity and reducing waste generation from those specific steps are key areas for investment from a sustainability perspective.
You can derive a practical translation of the environmental impact of bioprocessing based on a “generic” biological DS (BDS) process. The global warming potential of a biologic produced in a bioprocess based on single-use technology (SUT) in the United States is 22.7 tons CO2 equivalent per 1 kg BDS (10). That’s equivalent to the annual CO2 emissions of five automobiles (11). Extrapolating those values to an overall industrial monoclonal antibody (mAb) production of 25 metric tons is the CO2 emissions equivalent of 5208 cars (12). A more visionary way to think of that is to consider the amount of CO2 produced for 200 kg of all 92 approved mAbs: the equivalent emissions of 456 trips around the world in an average car!
After electricity, the second major contributor to overall environmental impact of bioprocessing is related to single-use equipment, with a total of 769 kg estimated to be consumed per batch (10). Some materials can be recycled, but that process can be difficult to implement because product-contacting components require cleaning and deconstruction (expending labor and energy resources) before entering the recycling/reuse supply chain.
To drive change forward, it is essential to approach the topic of sustainability holistically. Herein, we propose three relevant high-level topics that require innovation in the biopharmaceutical industry: metrics, process-development strategies, and supply-chain innovation. The examples below suggest how such innovations could help drive bioprocesses toward sustainability.
Metrics: Quantification is necessary for assessing a current state and for benchmarking progress in the right direction. The metrics used to quantify sustainability-related parameters should be easy to use, meaningful, and truly representative of the sustainability implications of biologic manufacturing processes. The following example specifically highlights the limitations of using PMI calculation as the sole basis for environmental impact assessment of a process, highlighting the need for a more comprehensive alternative metric to assess the entire environmental impact of a process or technology. Some intriguing and somewhat counterintuitive results come from applying a simplified PMI assessment to compare production of 200 kg mAb DS in a specific year by a traditional (low-PMI) intensified fed-batch process with that of a continuous process (13).
For comparison purposes, we keep the bioreactor production scale, duration, and number of downstream steps fixed. The major difference that arises between the two options is higher productivity in the continuous process (1 g/L/day) over that of the intensified fed-batch (0.2 g/L/day). Table 2 summarizes the comparison. Although the traditional process has a lower PMI, its productivity per unit time spent is multifold lower than that of the continuous process. Spending less time in the manufacturing plant to meet overall product demand provides a multifold reduction in electricity and other resources consumed through implementation of the continuous process. Hence, although the continuous process might appear less attractive based on solely a PMI assessment, it actually has a markedly lower environmental impact based on markedly higher productivity.
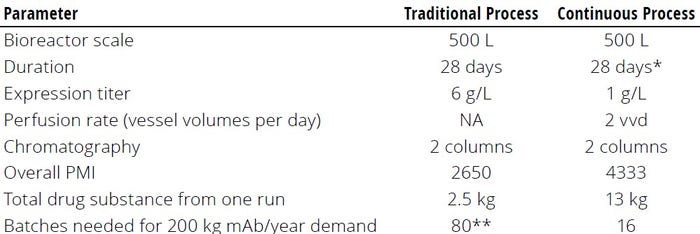
Table 2: Comparing a low–process-mass-intensity (low-PMI) intensified fed-batch
process with a continuous process for monoclonal antibody (mAb) production.
* Varies based on process duration
** Theoretical exercise; in practice, a large-scale bioreactor would be
used to meet demand, which would increase the facility footprint and electricity demand.
Process-Related Innovations: Biomanufacturing processes produce and supply life-saving drugs to patients in need. Innovations to optimize productivities of such processes both improves cost of goods (CoG) for biopharmaceutical companies and helps to improve their sustainability. Some innovations in this industry could be geared toward rethinking existing technologies to enhance their applications; others could provide dramatic and disruptive changes in the way that we approach process research and development.
Perfusion Cultures: Continuous biomanufacturing promises to usher in a new era of cost-effective BDS production through improved productivity and facility efficiencies. Perfusion cultures are an essential component of this concept, but they demand large volumes of culture media to maintain culture performance and productivity. Typically, the remaining volume of a permeate stream in a perfusion bioreactor containing both product and spent media is discarded as waste after initial product capture. That can account for significant loss of water and nutrients that otherwise could be reused in the same process. We demonstrated the feasibility of recycling spent media back to a bioreactor, recapturing some nutrients that otherwise would be discarded as waste while simultaneously improving the material efficiency of manufacturing (14).
To demonstrate proof of concept for permeate recycling, we used a deep-well–plate mock perfusion model and Sartorius Ambr bioreactors in an “off-line” mode with permeate sourced from an independent larger-scale culture. Thus, we could examine the culture performance with recycled material sourced from different stages of perfusion. However, the ultimate vision for this recycling strategy would be to recycle permeate within the same run (Figure 3). A key challenge with such a set-up would be continual accumulation of inhibitory by-products in the bioreactor over time. Intermittent breaks could be implemented as a strategy for clearing those by-products — and the timing and duration of such breaks will be the subject of future studies. Identification and analysis of those compounds could enable rational development of more robust perfusion media.
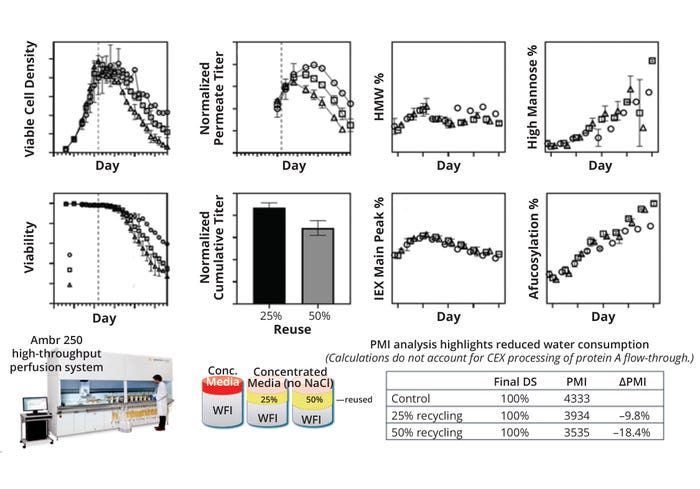
Figure 3: Overview of the environmental impact of permeate-reuse feasibility on process mass intensity (PMI), adapted from (14). CEX = cation-exchange ( chromatography), HMW = high molecular weight, IEX = ion-exchange ( chromatography), WFI = water for injection.
A PMI exercise shows that an 18.4% improvement in material efficiency could be achieved with a 50% permeate recycle rate, assuming no change in productivity. For a commercial mAb process serving a sizable market demand of 200 kg/year, that translates to 1515 metric tons of water for injection (WFI) saved over 10 years (assuming that water contributes to 95% of total PMI). Such results demonstrate the technical feasibility of recycling permeate in a perfusion process. We acknowledge that this is just a first step. Additional work is needed on several fronts to make the strategy practical for the manufacturing floor. However, our study should help to spur further innovation in continuous biomanufacturing.
Harvest and Primary Recovery: Using depth filtration for primary recovery is another way to connect sustainability with innovation. Historically, this unit operation has been approached by experimentally determining process performance metrics (e.g., filter loading, pressure drops, turbidity, and binding capacities) with empirical correlations to scale-up and membrane variability. The resulting processes must balance variability in performance (when operated at target loadings) against robustness (when operated at reduced loadings with filter oversizing). Neither option is desirable in terms of process robustness/scale-up or environmental impact.
Hence, an opportunity clearly presents itself for us to take a rational, prospective approach based on first principles and mechanistic modeling. A potential solution could be achieved by investment in a fundamental understanding of membrane/filtration mechanisms to improve filter efficiency. Deconvolution of such a complex problem into discrete elements would require understanding flow distribution in the absence of particulates, including dye-binding and computational fluid dynamics (CFD) studies. Investigations would follow into adsorption phenomena (15), the fundamentals of particle separation, changes in flowpaths as a function of fouling, and foundations of scale-down modeling.
As Figure 4 illustrates, significant progress has been achieved through innovative advancements and CFD applications to unique depth-filter geometries without the presence of particles (16). Such advanced tools were leveraged to assess flow distributions and the potential for flow nonuniformities in Cytiva’s Supracap PDH4 depth filters. Instead of an equal flow distribution among the four paths, CFD illustrated flow nonuniformities (Figure 4a), which were corroborated by dye-based experimental results (Figure 4b). The data suggest that parts of the depth filter were not sampled because of flow anomalies that led to filter-area underuse. Additional CFD results (Figure 4c) provided a three-dimensional (3-D) view of the flowpaths and stream lines in different zones of the filter. In a manner adapted from Nejatishahidein et al. (17), targeted experiments were used to assess the depth-filter capacity using marker proteins (homologous to host-cell proteins) and nucleic acids (Figure 5). Residence-time distribution analyses subsequently were completed to confirm modeling results.
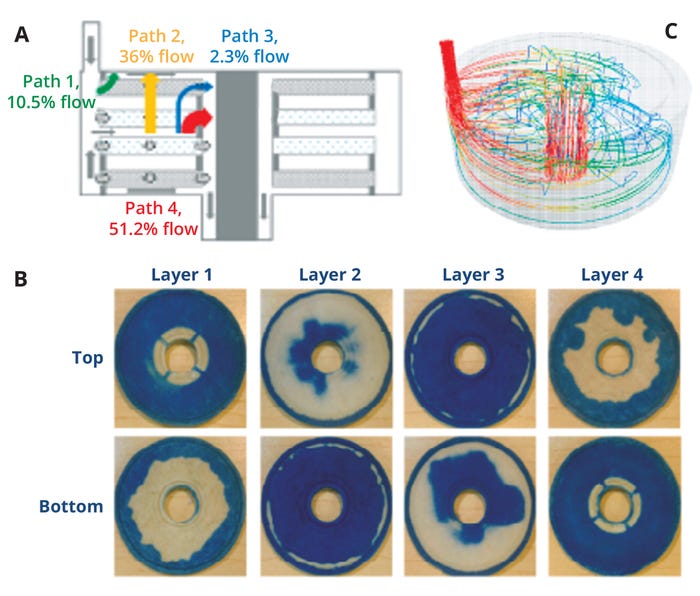
Figure 4: Flow distribution determined by pressure-flow computational fluid dynamics (CFD) analyses (a), dye binding (b), and CFD (c) for a depth filter (without particulates), illustrating flow-path distributions (28).
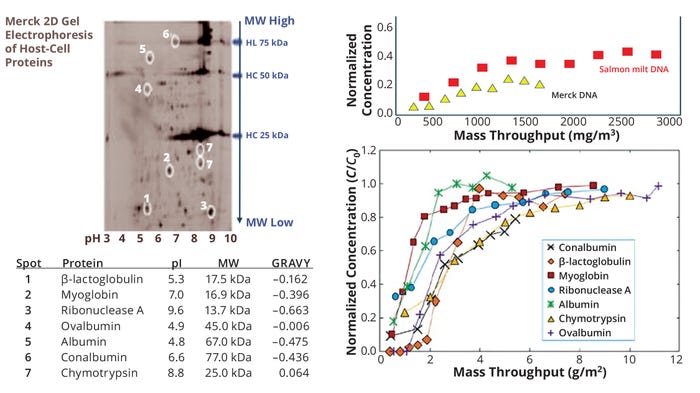
Figure 5: Breakthrough curves for proteins and nucleic acids as a function of membrane loading (28). GRAVY = index of average hydrophobicity and hydrophilicity, HC = heavy chain and constant region, HL = heavy and light chain, MW = molecular weight.
Combining those independent fundamental approaches provides a mechanistic approach to design and scale-up of depth filters for primary recovery based on first principles. This approach helps to improve sustainability by reducing the need for filter oversizing and associated waste generated during filter manufacturing and postuse disposal. Recent research has demonstrated the benefits of hybrid models — combining bench-scale testing with quantitative structure–activity relationship (QSAR) models — to predict depth-filtration performance as a function of scale-up based on feed properties (18). Retrospective evaluation of existing technologies using advanced computational tools can help us to gain insights that are not attainable readily through experimentation. Insights gained from such modeling efforts will be useful for identifying opportunities to develop new technologies with significantly reduced environmental footprints. The industry can and should apply an overall quantitative assessment of each new technology encompassing all of those key factors against existing available or alternative technologies. Development of such a quantitative metric is currently underway within the NIIMBL sustainability workstream.
A disruptive approach to consider in terms of sustainability is replacing experimentation with modeling where appropriate. The concept of in silico development of chemistry, manufacturing, and controls (CMC) operations has been proposed (19). One application of the concept was demonstrated by Simon DiMartino at the University of Edinburgh in Scotland, using machine learning (ML) to optimize the morphology of stationary phases (20, 21). Figure 6 provides a specific example for the translation of an abstract concept to reality. Such an approach could be extended to other bioprocessing fields including systems biology for testing specific metabolic pathways, mechanistic modeling of chromatography (in lieu of experimentation), and biophysical modeling, either to ascertain the effects of specific sequential changes on chromatographic retention or to assess the potential for separating specific impurities using different chromatographic modes (22).
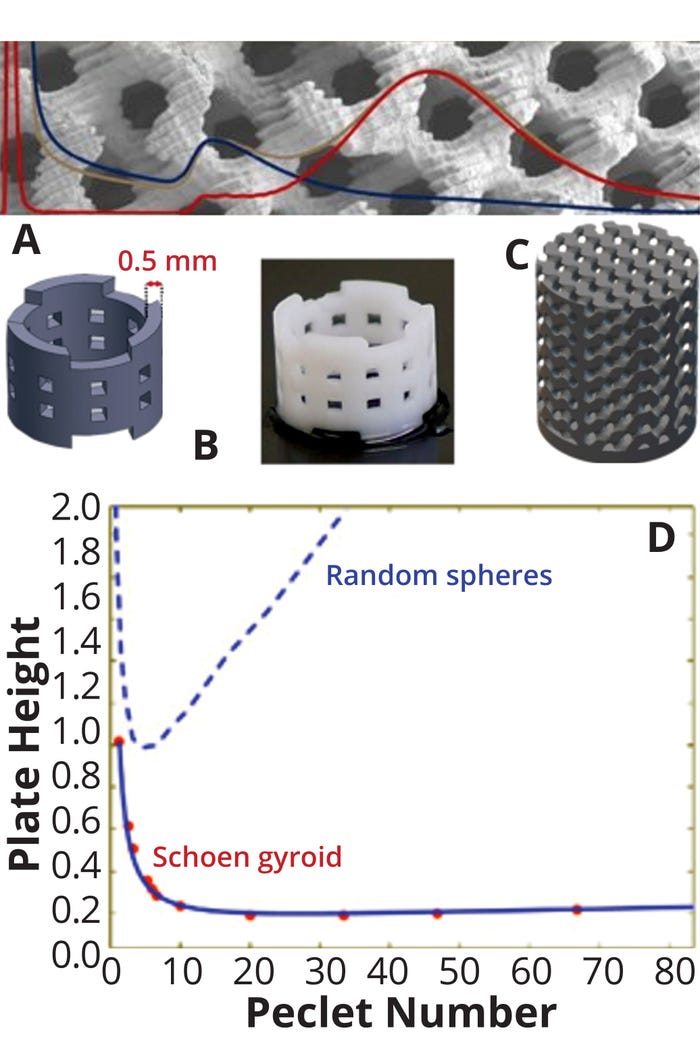
Figure 6: (a) Application of modeling to optimize chromatographic packing and operations (18); (b) computer-aided design (CAD) model and 3-D printed hollow cylinders; (c) CAD and 3-D printed gyroid; (d) comparing reduced plate height with Peclet number (Pe, the ratio of advective to diffusive transport in packing) for random spheres and Shoen gyroids illustrates the markedly improved performance with the latter (17). A Shoen gyroid is a mathematical model describing complex structures with high surface area. Note markedly improved performance based on the lower reduced plate height (representing better column packing) as a function of Pe: The higher the Pe value, the lower the contribution of diffusive transport, a common rate-limiting phenomenon in chromatographic processes.
Supply-Chain Innovations: The global healthcare industry is responsible for 4–5% of global emissions (23). In industrial nations, that rises to 10% of national emissions, with the pharmaceutical industry making up around 20% of all healthcare industry emissions.
Scope 1 emissions are direct emissions from sources owned or controlled by a reporting organization, such as emissions from combustion in owned or controlled boilers, furnaces, vehicles, and chemical manufacturing processes (24, 25). Scope 2 emissions are indirect emissions associated with the generation of purchased energy, such as public utilities producing electricity, steam, heating, and cooling. Scope 3 emissions are the result of activities from assets not owned or controlled by a reporting organization, but which the organization affects indirectly through its value chain. Scope 3 includes all sources not within an organization’s scope 1 and 2 boundaries; such emissions for one company might be scope 1 and 2 emissions for other entities.
Also referred to as value-chain emissions, scope 3 often represents the majority of a given organization’s total GHG emissions (25). In the biopharmaceutical industry, scope 1 and 2 GHG emissions usually are significantly smaller by proportion. Although scope 1 and 2 emissions typically are captured well and under the full control of a given company, scope 3 emissions are not well understood. That indicates the need for a collaborated effort among manufacturers and suppliers to work toward a common goal of understanding and reducing the overall footprint of the biomanufacturing supply chain. Below are two examples of innovations emerging as supply-chain opportunities for sustainable innovation.
SUT suppliers are working to reduce the amount of disposable components associated with single-use and continuous processing — bioprocessing bags in particular. Sartorius AG’s corporate research department has collaborated with Sanofi and Veolia to demonstrate proof of concept for recycling Flexsafe bags, for which their film was designed (26). Because most buffers and media are not hazardous fluids, used bags could be recycled. The material of construction of such bags and their associated connections are well characterized (Figure 7). The conceptual study showed that the resulting recycled plastics exhibit physical properties similar to equivalent virgin materials (26). Based on quantities available for recycling, the cost and efficiency of sorting to maintain material value are other areas that need to be addressed further for implementation of this strategy.
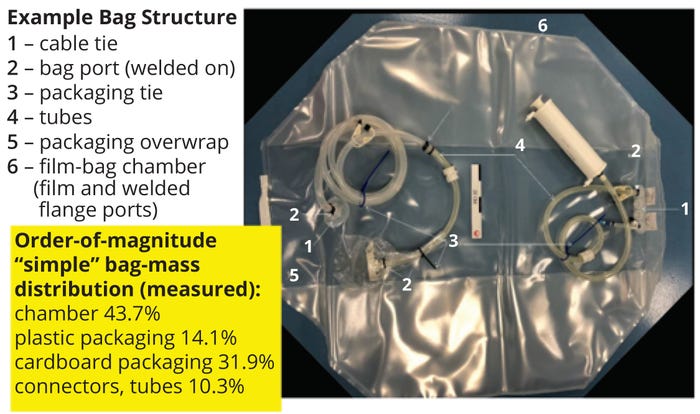
Figure 7: Overview of Sartorius proof of concept for recycling single-use bags (adapted with permission); bioprocessing bags used for media and buffer preparation/ storage represent the largest amount of plastic waste. Most buffers and media are not hazardous fluids, and the Flexsafe bag chamber is designed to be recyclable with well-known and characterized materials: polyethylene vinyl alcohol (PVOH), low-density polyethylene (LDPE), linear lowdensity polyethylene (LLDPE), polyamide (PA), and thermoplastic elastomer (TPE).
Packaging Materials: Another example relates to packaging that is required for controlled-temperature storage and protecting materials and components during shipping. One question concerns the optimal location of production to reduce overall environmental impact associated with shipping/transportation. Cytiva is developing an alternative approach to shipping based on a goal of reducing the use of a polystyrene, a nonrenewable and difficult-to-recycle material. Figure 8 overviews the comprehensive approach from establishment of quantifiable targets to optimization of a supply-chain network.

Figure 8: Overview of Cytiva’s disruptive innovation for sustainability imperatives in a global packaging and shipping initiative (adapted with permission).
Disruptive Innovation: We Are Up to the Challenge
An excellent opportunity has arisen to evaluate and rethink existing technologies with a focus on sustainability. One challenge has been quantifying the impact of bioprocessing on the environment to find specific targets for development. Recent advances have helped the industry begin to categorize its impacts (10). Such an assessment can incorporate both retrospective and prospective approaches.
The first perspective involves developing technologies with the same or better functionality as that of established methods but with reduced environmental footprints. Such a retrospective approach might focus on recapitulation of a technology’s functionality with a reduced environmental footprint (e.g., using sustainably sourced plastics). The second perspective aims to create entirely new approaches leveraging enhanced understanding of first principles that has arisen since the debut of an initial technology. Specific examples include the development of biosimilars such as insulin (22), in which marked improvements in efficiency have been applied to a range of unit operations by leveraging decades of learning and technology advancement.
Optimization of unit operations in process development will provide only a fraction of the necessary sustainability improvements. Replacement of existing methods with pioneering technologies that incorporate sustainability perspectives is achievable through innovation. Collaboration among all key stakeholders is essential to defining the opportunities, translating them into business propositions, and implementing the resulting technologies throughout the supply chain. One already apparent issue is clarity about who those key stakeholders are. As in Table 2, cursory or initial assessment of an opportunity by a small group of stakeholders could lead to missed opportunities. Broader coalitions of stakeholders including technology end users will help to identify opportunities for new technologies with enhanced sustainability and encourage technology suppliers to produce them. The resulting value proposition is markedly different as an impetus to embrace change.
Conclusions: Some key opportunities have been identified to improve bioprocess sustainability — including a goal to evaluate PMI and reevaluate operational efficiencies for existing bioprocesses (commercial and clinical production). An identified gap that is being addressed is the need for a comprehensive sustainability metric for biologics. These efforts are essential to translating the abstract concepts of environmental impact to tangible applications of engineering and scientific approaches that will improve sustainability. Some intermediate innovation strategies include improvements in process productivity — e.g., reprocessing and reusing spent media from continuous manufacturing processes (with the first steps toward proof of concept having been completed), development of alternative chromatography systems for targeted purification, and depth-filtration technological improvements that leverage first principles and advancements in CFD.
It’s not easy to translate environmental sustainability goals into financial decisions — beyond the global and philosophical questions related to the value of emitting a ton of CO2. What is the bioprocess/biomanufacturing community willing to pay for products with reduced environmental footprints? For example, if a new product or technology has equivalent functionality to an existing option with a fraction of the environmental impact, how much of a premium could that new alternative demand? The feasibility of decision-making about locations for manufacturing or distribution networks based on existing sustainability metrics has been demonstrated. As bioprocess manufacturers have adopted a global manufacturing footprint with limited resources (e.g., water and energy), disruptive innovation will be imperative to overcome those resource and environmental constraints. One excellent example has been the development of alternatives to replace the formerly ubiquitous Triton X-100 surfactant once its deleterious environmental impact was appreciated. Multiple green-chemistry approaches were identified, including the use of sustainable feedstocks (rather than petroleum-based substrates). Such investigations led to a range of new surfactants such as tetradecyl-dimethylamine oxide (TDAO) and Simulsol SL 11W detergent, which are now widely available to the bioprocessing community (27).
But how much of a premium is the industry willing to pay for achieving its sustainability targets? To address that question, we need a cross-industry consortium to drive and enable related innovations and help to develop the necessary tools and metrics. Maybe a new financial model will be necessary to incentivize that approach. A unified approach may evolve in which companies include sustainability metrics on their products in their annual reports. Industry leaders such as MSD, Cytiva, and Sartorius already include their progress toward sustainability targets in their reporting.
Application of machine learning and advanced modeling techniques could help the industry to reduce its experimental burden to both increase process robustness and decrease the environmental impact of bioprocessing, thus improving its sustainability. The current state of process development and manufacturing is unlikely to be adequate for supporting global access to medicines. Hence, applying constraints to resources will demand identification of opportunities for improvement and innovation. Although the sustainability goals proposed herein might seem daunting or audacious, the bioprocessing community was founded and continues to flourish based on innovation and leadership — and those are both key to achieving sustainability.
Acknowledgments
We are grateful for insightful discussions and contributions from Jack Gavin (in environmental compliance, sustainability, and stewardship, part of the process research and development group at MSD); the American Chemical Society’s Green Chemistry Institute Pharmaceutical Roundtable (GCI PR); the Biopharma Focus Group within the sustainability workstream of the National Institute for Innovation in Manufacturing Biologics (NIIMBL); Konstantin Zoeller (Novartis); Anna Grönberg, Linus Laurin, and Ryan Walker (Cytiva); and Magali Barbaroux, Matteo Alaria, and Jonathan Cutting (Sartorius).

ABSTRACT
The abstract of this article was published in BPI’s September 2024 eBook, Sustainable Facilities: Emerging Approaches to Design and Construction, which can be downloaded here: https://www.bioprocessintl.com/facility-design-engineering/ebooksustainable-facilities-emerging-approachesto-design-and-construction.

References
1 Sawyers CL. Herceptin: A First Assault on Oncogenes That Launched a Revolution. Cell 179(1) 2019: 8–12; https://doi.org/10.1016/j.cell.2019.08.027.
2 Gardner J. A Decade of Cancer Immunotherapy: Keytruda, Opdivo, and the Drugs That Changed Oncology. Biopharma Dive 4 September 2024; https://www.biopharmadive.com/news/cancer-immunotherapy-decade-keytruda-opdivo-pd1-oncology/725774.
3 Jain S, et al. Messenger RNA-Based Vaccines: Past, Present, and Future Directions in the Context of the COVID-19 Pandemic. Adv. Drug Deliv. Rev. 179, 2021: 114000; https://doi.org/10.1016/j.addr.2021.114000.
4 Barbaroux M, et al. The Green Imperative: Part One — Life-Cycle Assessment and Sustainability for Single-Use Technologies in the Biopharmaceutical Industry. BioProcess Int. 18(6) 2020: 12–19; https://www.bioprocessintl.com/single-use/the-green-imperative-part-one-life-cycle-assessment-and-sustainability-for-single-use-technologies-in-the-biopharmaceutical-industry.
5 Madabhushi SR, et al. Quantitative Assessment of Environmental Impact of Biologics Manufacturing Using Process Mass Intensity Analysis. Biotechnol. Prog. 34(6) 2018: 1566–1573; https://doi.org/10.1002/btpr.2702.
6 Budzinski K, et al. Introduction of a Process Mass Intensity Metric for Biologics. New Biotechnol. 49, 2019: 37–42; https://doi.org/10.1016/j.nbt.2018.07.005.
7 Annual Energy Outlook 2021 with Projections to 2050. US Energy Information Administration: Washington, DC, 3 February 2021; https://www.eia.gov/outlooks/aeo/pdf/00%20AEO2021%20Chart%20Library.pdf.
8 Sustainability. Merck & Co., Inc.: Rahway, NJ, USA, 2024; https://www.msd.com/company-overview/sustainability.
9 Whitford W. Single-Use and Sustainability: Continued Studies Using LCA Tools. Single-Use Technologies III: Scientific and Technological Advancements (Snowbird, UT, 23–26 September 2018). Engineering Conferences International: New York, NY; https://dc.engconfintl.org/sut_iii/36.
10 Budzinski K. et al. Streamlined Life Cycle Assessment of Single Use Technologies in Biopharmaceutical Manufacture. New Biotechnol. 68, 2022: 28–36; https://doi.org/10.1016/j.nbt.2022.01.002.
11 Greenhouse Gas Emissions from a Typical Passenger Vehicle. US Environmental Protection Agency: Washington, DC, 23 August 2024; https://www.epa.gov/greenvehicles/greenhouse-gas-emissions-typical-passenger-vehicle.
12 Ecker DM, Jones SD, Levine HL. The Therapeutic Monoclonal Antibody Market. mAbs 7(1) 2015: 9–14; https://doi.org/10.4161/19420862.2015.989042.
13 Madabhushi SR, Pinto NDS, Lin H. Comparison of Process Mass Intensity (PMI) of Continuous and Batch Manufacturing Processes for Biologics. New Biotechnol. 72, 2022: 122–127; https://doi.org/10.1016/j.nbt.2022.11.002.
14 Madabhushi SR, et al. An Innovative Strategy To Recycle Permeate in Biologics Continuous Manufacturing Process To Improve Material Efficiency and Sustainability. Biotechnol. Prog. 38(4) 2022: e3262; https://doi.org/10.1002/btpr.3262.
15 Khanal O, et al. Contributions of Depth Filter Components to Protein Adsorption in Bioprocessing. Biotechnol. Bioeng. 115(8) 2018: 1938–1948; https://doi.org/10.1002/bit.26707.
16 Kim M, et al. Flow and Residence Time Distribution in Small-Scale Dual-Layer Depth Filter Capsules. J. Memb. Sci. 617, 2021: 118625; https://doi.org/10.1016/j.memsci.2020.118625.
17 Nejatishahidein N, et al. E��ffectiveness of Host Cell Protein Removal Using Depth Filtration with a Filter Containing Diatomaceous Earth. Biotechnol. Prog. 36(6) 2020: e3028; https://doi.org/10.1002/btpr.3028.
18 Liu P, et al. Combining Descriptive and Predictive Modeling To Systematically Design Depth Filtration-Based Harvest Processes for Biologics. Biotechnol. Bioeng. 121(9) 2024: 2924–2935; https://pubmed.ncbi.nlm.nih.gov/38837221.
19 Roush D, et al. Toward In Silico CMC: An Industrial Collaborative Approach to Model-Based Process Development. Biotechnol. Bioeng. 117(12) 2020: 3986–4000; https://doi.org/10.1002/bit.27520.
20 Dimartino S, et al. Flexible Material Formulations for 3D Printing of Ordered Porous Beds with Applications in Bioprocess Engineering. Bioresour. Bioprocess. 9(1) 2022: 20; https://doi.org/10.1186/s40643-022-00511-9.
21 DiMartino S. Machine Learning for Morphology Optimization of Perfectly Ordered Stationary Phases. 5th Modeling workshop (5MW): June 2023, Favrholm, Denmark. Precision Meetings and Events: Washington, DC.
22 Roush D, et al. Insulin Purification: Innovation Continuum Via Synthesis of Fundamentals, Technology, and Modeling. Biotechnol. Bioeng. 121(8) 2024: 2409–2422; https://doi.org/10.1002/bit.28427.
23 Beatie KM, et al. Challenges for Net Zero Carbon Pharmaceutical Manufacturing. Pharm. Eng. March–April 2023; https://ispe.org/pharmaceutical-engineering/march-april-2023/challenges-net-zero-carbon-pharmaceutical-manufacturing.
24 Kansteiner F. Setting Sights on Suppliers: How Biopharma Is Tackling the Environment in Its ESG Commitments. Fierce Pharma 19 July 2022; https://www.fiercepharma.com/pharma/setting-sights-beyond-scope-1-and-2-how-biopharma-tackling-environment-its-esg-commitments.
25 Philpott J. Pharma’s Path to Net Zero: Targeting Scope 3 Emissions. Pharm. Technol. 29 November 2023; https://www.pharmaceutical-technology.com/features/pharmas-path-to-net-zero-targeting-scope-3-emissions.
26 Luu D-N, et al. Recycling of Post-Use Bioprocessing Plastic Containers: Mechanical Recycling Technical Feasibility. Sustainability 14(23) 2022: 15557; https://doi.org/10.3390/su142315557.
27 Straub JO, Shearer R, Studer M. Rational Selection of Alternative, Environmentally Compatible Surfactants for Biotechnological Production of Pharmaceuticals: A Step Toward Green Biotechnology. Environ. Toxicol. Chem. 33(9) 2014: 2140–2146; https://doi.org/10.1002/etc.2660.
28 Roush D, et al. Reducing the Bioprocessing Environmental Footprint and Saving Lives Via Advanced Engineering Principles. Convergence: Connecting Membrane Science with Societal Challenges. North American Membrane Society: Estes Park, CO, 2021.
Formerly with MSD, David Roush is now chief executive officer of Roush BioPharma Panacea, LLC, in Colts Neck, NJ; [email protected]. Sri Ranganayaki Madabhushi is principal scientist and director of process research and development at MSD ([email protected]).
Herceptin is a registered trademark of Genentech (a member of the Roche Group), and Keytruda is a registered trademark of Merck & Co., Inc., Rahway, NJ, USA. Ambr and Flexsafe are registered trademarks of Sartorius AG. Supracap is a registered trademark of Cytiva.
You May Also Like





