WWW.LIFETOUCH.COM
So many manuscripts we receive begin by mentioning the “ever-growing” or “ever-expanding world of biopharmaceuticals.” For anyone who’s been covering this industry for a while, that’s an understandable impression — but it’s not always easy to express. Despite increasing in maturity, that “world” remains on the cutting edge of science and technologies for innovative therapeutic development, and its entrepreneurial spirit remains strong.
But we’re all accessing information in different ways now than in the past. We need and want that information quickly, we want assurance of its timeliness, and we want to trust that interpretations are focused accurately on our needs. Only then can BPI truly live up to its tag line:
Covering the whole development process
…. A good portion of news releases arriving in my inbox are meant for people outside our industry or in areas that BPI does not cover. If we have to sift through all of that, I imagine that many of you do too.
When first designing BPI, Ch...
Welcome New BPI Colleague
In April,
BioProcess International
waved a reluctant goodbye to European strategic marketing consultant Joanna Hendrikx. Pregnant with her second child, she is looking forward to devoting herself exclusively to motherhood. We will miss her energy, finesse, and sense of humor even as we welcome our new colleague taking over her role here.
Victoria Biscoe
has a long history with Informa, our parent company, having joined in 2010 as a key account manager for the Informa Life Sciences conference division in Europe. In 2012, she moved to the Pharmaceutical Training Institute division as a customized training consultant. That led to her role as head of customized training for Knect365 Learning in 2015 — and ultimately to BPI.
Before Informa, Victoria was a recruitment consultant for Acme Appointments in London. She has a bachelor of science degree in psychology from Loughborough University, where she also played netball on a national college-sports champion team. BPI is excited to w...
ADOBE STOCK (HTTPS://STOCK.ADOBE.COM)
Drug packaging is subject to a number of regulatory requirements, including those for product containers and packaging. For example, according to the federal Food, Drug, and Cosmetic Act (FD&C) section 501(a)(3), a drug is considered adulterated “if its container is composed, in whole or in part, of any poisonous or deleterious substance which may render the contents injurious to health.” And 21 CFR states that drug packaging “shall not be reactive, additive, or absorptive so as to alter the safety, identity, strength, quality, or purity of the drug beyond the official or established requirements.”
Such requirements are enforced by the US Food and Drug Administration (FDA). The agency has produced guidance on submitting an application for a new drug product, focusing specifically on the information required about the packaging materials:
Guidance for Industry: Container Closure Systems for Packaging Human Drugs and Biologics
(
1
). It states that an application must...
STORYBLOCKS (WWW.STORYBLOCKS.COM)
Host-cell proteins (HCPs) constitute a significant class of process-related impurities during biologics manufacturing. Due to their potential impact on product quality and efficacy as well as patient safety, the total amount of residual HCP in a biological drug substance generally is considered a critical quality attribute (CQA) that usually needs to be tested for during batch release (
1
,
2
). It is both an “industrywide” common understanding and a regulatory requirement to remove HCPs from biologics to acceptably low levels that will not affect product quality, compromise expected product efficacy, or jeopardize patient safety (
3
,
4
). However, the complexity and diversity of residual HCP composition in drug substances and limited knowledge publicly available on the biological, physiological, pharmacological, and toxicological effect of individual HCPs detected in biologics create significant challenges. The main difficulty is in effectively determining at what lev...
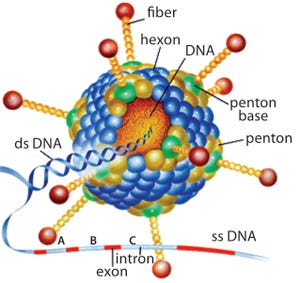
Replication-defective (E1 gene-deleted) chimpanzee type-3 adenovirus (ChAd3) (HTTPS://MICROBEWIKI.KENYON.EDU/INDEX.PHP/ ADENOVIRUS-BASED_GENE_THERAPY:_A_PROMISING_ NOVEL_CANCER_THERAPY)
In December 2013, a two-year-old child in Guinea became the first person to be killed by Ebola in the most recent outbreak. In March of the following year, that outbreak was declared in West Africa. By mid-2014, the World Health Organization (WHO) had declared it to be a public health emergency of international concern and urged pharmaceutical companies to accelerate their development of candidate vaccines. At the peak of the outbreak in 2014, more than 1,200 new cases of Ebola were reported every week in Liberia, Sierra Leone, and Guinea. This was the most significant outbreak in Africa during the past 50 years.
In acknowledgment of WHO’s request, GSK initiated an Ebola project and gathered staff with relevant expertise to push vaccine candidates from laboratory scale to a large-scale product. The selected product candida...
ADOBE STOCK (HTTPS://STOCK.ADOBE.COM)
Successful development of liquid biopharmaceutical formulations requires careful assessment of the biophysical properties of the protein in solution, primarily focused on achieving optimal conformational and colloidal stability of the drug-substance molecule (
1
–
11
). It also involves extensive stability studies under stressed conditions. Using state-of-the-art biophysical tools for characterization of developed products, those studies are based on key biophysical descriptors and extended particulate characterization methods (subvisible particles in micro- and nano-size range) to deliver a stable product for market with a shelf life of about two years (
12
–
15
).
Because of potential immunogenic risk associated with the presence of proteinaceous particles, comprehensive particle characterization in macromolecular drug-product development has drawn greater attention from the biopharmaceutical industry and regulatory agencies around the globe (
16
–
23
). To help red...
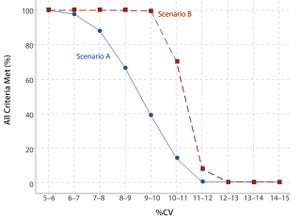
Figure 1: Likelihood meeting all criteria per scenario
Analytical linearity as well as assessments of precision and accuracy determine the range for a bioassay (
1
). USP <1033> recommends comparing confidence intervals (CIs) against target validation acceptance criteria in a bioassay validation exercise, but there are no clear guidelines for determining the criteria (
2
). Here I address several aspects of a bioassay validation, namely
CIs for the first four parameters (
R
2
, slope, intercept, and %RB) can be directly related to %CV. Doing so provides information on the likelihood of passing a set of validation acceptance criteria for a given level of bioassay precision.
Confidence Interval Calculations
To align with the bioassay validation example in USP <1033> the following assumptions are made:
Linearity is shown if the slope is equivalent to one and the intercept is equivalent to zero (there also might be a criterion on
R
2
). CIs for the intercept and slope can be calculated using Equations 1 and ...
Biologic drugs are a driving force in the pharmaceutical industry. More than 2,700 were in pharmaceutical pipelines as of June 2017 to treat cancers (836), rare diseases (566), neurologic conditions (420), autoimmune disorders (311), and other ailments (567) — triple the number in 2013 overall (
1
). Biopharmaceuticals make up more than half of all drugs in development and are nudging out traditional small-molecule drugs rapidly while already bringing in billions of dollars in sales.
The success of such drugs is predicated on their ability to treat disease targets better than existing/classical pharmaceuticals and with fewer side effects. But that alone may not be sufficient to achieve the outcomes upon which reimbursement decisions increasingly depend. According to Deloitte, the drug itself represents a diminishing share of what comes together to deliver an overall patient outcome (
2
).
Subcutaneous Biologics
Biologics present several challenges that could limit their reach and potential. First, they a...
Great strides have been made in increasing the yield of biotherapeutic proteins mainly through process improvements with a focus on media and feed strategies. But aside from single-gene knockouts to allow for metabolic selection systems, the Chinese hamster ovary (CHO) host cell line remains largely unchanged from what was used 30 years ago. In a webinar on 21 March 2018, Jamie Freeman (product manager at Horizon Discovery) focused on this as a bioproduction application of clustered regularly interspaced short palindromic repeats (CRISPR) screening technology.
Freeman’s Presentation
A few years ago, Horizon realized the need for making cutting-edge CHO cells accessible to companies of all sizes. Starting with an existing industry-standard platform — the glutamine synthetase (GS) knockout approach to metabolic selection — the company built a GS knockout. Adventitious-agent testing and good manufacturing practice (GMP) banking of the cell line were complete in 2016. Since then, more than 20 companies have l...