
As demands increase for antibody therapeutics from both patients and regulators, new modalities arise that require critical analysis and multiattribute methods. This month's Featured Report highlights developments in antibody manufacturing, from artificial intelligence (AI)-assisted protein engineering to production, purification, and characterization.
A Fully Single-Use Downstream Process for Monoclonal Antibodies
The past decade has brought a rise in the use of disposable products for biopharmaceutical downstream processing, especially in chromatographic purification (1, 2). Applying such materials in bioprocessing reduces contamination risk, shortens operation times related to preparation and cleaning, and decreases operator error. As part of our investigation into the potential of single-use technologies in antibody manufacturing, we have evaluated the current generation of membrane chromatography that can operate at high flow rates to provide high productivity in a disposable system (3). In testing over several months, we reviewed this modern technology to understand better the impact that it can have on antibody purification processes and what it can offer as an alternative to resin-packed columns.
We compared manufacturing performance and other data from small- and manufacturing-scale protein A affinity membrane devices, and we sought to establish equivalency with column chromatography. We found that the membrane devices achieved 10× higher productivity than that of a standard protein A affinity column for purification of monoclonal antibodies (mAbs) from Chinese hamster ovary (CHO) cell-culture harvest at small scale. The manufacturing-scale device provided similar productivity levels. For removal of host-cell proteins (HCPs) and DNA (hcDNA), the single-use membrane devices showed similar or better performance than did the column. Finally, we performed a fully disposable mAb-purification process based on membrane capture. The process leveraged disposable materials for all vessels and flow paths, a disposable ion-exchange (IEX) membrane device, and a single-use tangential-flow filtration (TFF) membrane capsule at manufacturing scale. The IEX devices effectively removed impurities, and the process with fully disposable materials was demonstrated to purify antibodies efficiently in a short process time. Our results demonstrate that fully disposable downstream processes can purify mAbs just as effectively as traditional processes that many companies rely on today but in a competitive timeline at desired production scale.
EFFICIENCIES OF MEMBRANE CAPTURE
Materials and Methods: Protein A affinity membrane devices for mAb purification have been developed and released by a number of bioprocess component manufacturers (4). We compared the capture step using a protein A capture membrane from W.L. Gore & Associates and MabSelect SuRe resin from Cytiva, which is used in many biomanufacturing processes. The load material was a typical immunoglobulin G (IgG) at 1.7 g/L in clarified harvest fluid (CHF) from an upstream CHO process with a working volume of 500 L.
For the laboratory-scale experiment, we used a 3.5-mL Gore PROA102 protein A membrane device and a Cytiva 11003494 5-mL prepacked column; for production scale, we used two 1-L Gore PROA303 protein A membrane devices connected in parallel. Based on dynamic binding capacity (DBC) data obtained from previous experiments, we loaded the CHF at 20-mg mAb per mL of membrane volume at 24 seconds residence time (SRT) onto the membrane device and 30 mg per mL of resin at 180 SRT onto the resin column.
Tables 1 and 2 detail the chromatography steps and times for each scale and device. Whereas it took 133 minutes/cycle with the resin column, the time required per cycle with the membranes was under 10 minutes due to the high flow rate. Table 3 lists the amount of mAb purified by each device, with the number of cycles required and the total process time. Figure 1 shows mAb productivity in grams purified per liter of media over time. Although the 3.5-mL membrane required more cycles than did the 5-mL resin column, the former could purify more mAb material in less time because of its shorter time per cycle. The time required for 19 chromatography cycles to purify 805 g of mAb with two 1-L membranes was only three hours.
Figure 1: Monoclonal antibody (mAb) productivity per liter of protein A media per hour with each membrane device and resin-packed column
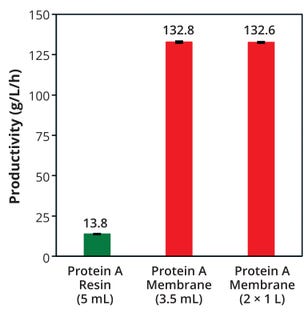
Results Comparing Productivity and Impurity Removal: The membrane devices had 10× higher mAb productivity than the resin column and comparable results even with larger membrane sizes. Yields from the protein A membranes were consistent from the first to the last cycle at both sizes, and results were equivalent or better than purification with a resin column (Figure 2).
Figure 2: Yield at each cycle with each protein A option; load mAb concentration was based on a biolayer interferometry (BLI) assay, and eluates were based on UV absorption at 280 nm. Average recovery for the resin is 102.0% (1.60 standard deviation), 106.1% (1.27 S.D.) for the 3.5-mL membrane, and 105.0% (0.90 S.D.) for two 1.0-L membranes.
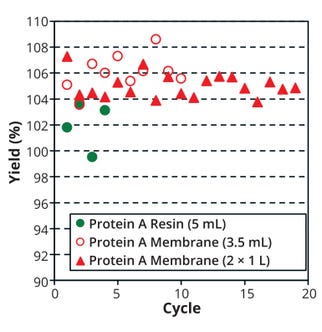
Figure 3 shows the volume of cycle eluates from each membrane device and column. When we compare eluate and media volumes at small scale, the membrane eluate was less than that from the column; it was comparable to that of the resin even at larger scales. Also, in the cycle range of our study, the volume of eluate per cycle remained consistent for all devices.
Figure 3: Eluate volume in each cycle for membrane devices and the resin-packed column; peak cut-off = 100–100 mAU (A280)
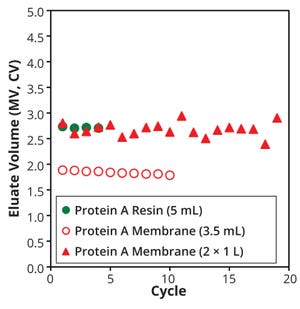
Figure 4 shows comparable leached protein A levels in eluent from each membrane device and column.
Figure 4: Leached protein A in eluate pools (left) from each device or column and (right) for each cycle using two 1-L membrane devices
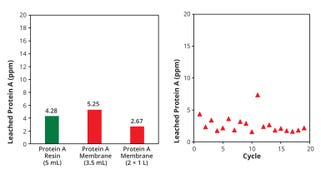
Values were comparable or lower than those reported for other protein A membrane devices and resin-packed columns (5, 6). In addition, there was no increasing trend for leached protein A through cyclic use with either membrane device (Figure 4b).
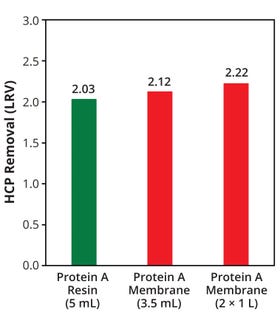
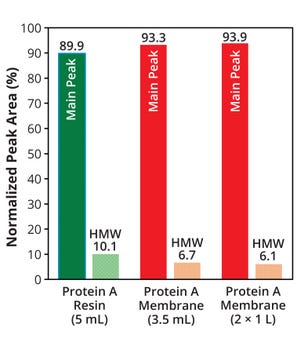
Figure 5 shows log reduction values (LRVs) relative to the load solution for HCPs in the eluate pools from each membrane and column. The membrane device efficiencies were comparable to or better than that of the column in terms of HCP removal. In addition, the membrane device was more effective at large scale, with a higher LRV than that of a typical protein A affinity capture process (5, 6). For hcDNA the LRV was 3.4 when using two 1-L protein A membrane devices. These results indicate that the membrane device is comparable to or more effective than conventional resin columns for removing impurities. Figure 6 compares peak percentages from a size-exclusion chromatography (SEC) assay for eluate pools from each device and column. The percentage of high–molecular-weight (HMW) species — typically aggregates and multimers — was lower in the membrane eluates than in the eluate from the resin-packed column.
Figure 5: Removal of host-cell proteins (HCPs) as represented by log reduction values (LRVs) for the eluate pool from each membrane device or column
In summary, we found that protein A affinity membrane devices were as effective as or better than conventional resin-packed columns for mAb purification. The membranes also provided much higher mAb productivity. Our results suggest that the membrane device is a very promising purification material for future mAb manufacturing.
Figure 6: Comparing the percentages of high– molecular-weight (HMW) species present in eluate from each membrane device or column
BENEFITS OF USING FULLY DISPOSABLE MATERIALS
Protein A affinity membrane chromatography is a promising technology for mAb manufacturing. We’ve discovered that using disposable material in bioprocessing can reduce contamination risk, operation time, and operator error.
Single-use purification materials that allow for fully disposable downstream processes — e.g., the IEX membrane devices, flow-path systems, and TFF membrane capsules mentioned above — have become widely available. That facilitates building fully disposable downstream processes based on membrane capture at manufacturing scale.
At laboratory scale, we established a fully disposable downstream mAb purification process using a Gore protein A membrane device with Sartobind Q anion-exchange (AEX) membrane device and Sartobind S cation-exchange (CEX) membrane devices (both from Sartorius) for a typical mAb produced by CHO cell culture. Table 4 details our results, demonstrating efficient removal of HCPs by the protein A membrane followed by the AEX/CEX membrane devices.
Having established that purification at laboratory scale, we moved on to demonstrate a fully disposable mAb production process with single-use materials in all downstream steps at manufacturing scale based on protein A capture with two 1-L protein A membrane devices as mentioned above. Table 5 lists the single-use devices used for each step, and Table 6 details the associated yields and mAb productivities. The AEX process gave a high mAb productivity of 189.2 g/MV/h, with high flow rates made possible by the membrane device. In addition, each process showed high step yields.
Table 7 summarizes the demonstration-run results of our fully disposable mAb purification process at manufacturing scale. Purification efficiency using membrane devices at manufacturing scale was comparable to that at the laboratory scale (up to the AEX chromatography step).
Our full manufacturing-scale run eliminated the CEX step, but with products that require further purification, our laboratory-scale results suggest that it is possible to use an CEX membrane device for that purpose.
FULFILLING THE PROMISE
Application of single-use materials in biomanufacturing is effective for reducing contamination risk and operational errors while reducing lead times and upfront investment as well as storage space associated with columns and membrane housings. We have established a fully disposable mAb production process to develop a new biopharmaceutical product and prepare it for clinical manufacturing.
For mAb purification, the protein A membrane device was comparable to or more effective than conventional resin-packed columns — with 10× higher productivity. Further, an IEX membrane device efficiently removed both HCPs and hcDNA. The overall mAb purification process based on single-use systems was as efficient at manufacturing scale as it was at laboratory scale. By using disposable equipment and membrane devices, we were able to remove the time and buffer necessary for column packing and associated preparation. These results demonstrate that a fully single-use mAb purification process — with disposable components in all steps — can achieve efficient purification as an effective tool for antibody drug-substance manufacturing.
Acknowledgments
We are grateful to Chase Snyder, Celia Hatfield, and Connor Young at AGC Biologics as well as Eric Van Voorhees, Mike McManaway, Jeffrey Cassel, and William C. Barrett, PhD, at W.L. Gore & Associates, Inc. for their support during our evaluations.
References
1 Samaras J, Micheletti M, Ding W. Transformation of Biopharmaceutical Manufacturing Through Single-Use Technologies: Current State, Remaining Challenges, and Future Development. Annu. Rev. Chem. Biomol. Eng. 13, 2022: 73–97; https://doi.org/10.1146/annurev-chembioeng-092220-030223.
2 Matte A. Recent Advances and Future Directions in Downstream Processing of Therapeutic Antibodies. Int. J. Mol. Sci. 23(15) 2022: 8663; https://doi.org/10.3390/ijms23158663.
3 Jacquemart R, et al. A Single-Use Strategy To Enable Manufacturing of Affordable Biologics. Compt. Struct. Biotechnol. J. 14, 2016: 309–318; http://dx.doi.org/10.1016/j.csbj.2016.06.007.
4 Osuofa J, Husson SM. Comparative Evaluation of Commercial Protein A Membranes for the Rapid Purification of Antibodies. Membranes 13(5) 2023: 511; https://doi.org/10.3390/membranes13050511.
5 Busse RA, et al. Sartobind Rapid A: Comparable High Product Quality as Protein A Resins at High Productivity. Bioprocess Online https://www.bioprocessonline.com/doc/sartobind-rapid-a-comparable-high-product-quality-as-protein-a-resins-at-high-productivity-0001.
6 KA1965230418AN. Capacity and Performance of MabSelect PrismA Protein A Chromatography Resin. GE Life Sciences: Chicago, IL, 2017; https://cdn.cytivalifesciences.com/api/public/content/digi-26524-original.
Corresponding author Shunsuke Shiina is principal downstream process development scientist, Neha Saxena is an analytical development scientist, and Rodrigo H. Gonzalez is senior director and head of the R&D Center at AGC Biologics in Bothell, WA; 1-425-485-1900; [email protected]; Recombinant antibody technology revolutionized biologics production, offering rapid, scalable, and cost-effective production. .
You May Also Like





