Elevating Your Pharmaceutical Facility to the Next Digital Plant Maturity Level
June 23, 2022
Pharma 4.0 technologies, offshoots from the Industry 4.0 model, focus on introducing new technologies for increased levels of digitalization within the pharmaceutical manufacturing industry. Many companies hesitate to embrace the Pharma 4.0 concept fully even though digitalization efforts are leading the way toward new levels of efficiency and productivity. The biopharmaceutical industry currently lags behind other industries in implementing digital technologies because of its rigorous and strict regulatory requirements.
Adopting Pharma 4.0 tools and concepts benefits manufacturers by harmonizing the flow of R&D VR data through manufacturing and distribution, strengthening cybersecurity, and improving quality while maintaining regulatory compliance. Technologies that help pharmaceutical manufacturers find new, innovative avenues to realize higher levels of connectivity and automation include artificial intelligence (AI), augmented reality (AR), virtual reality (VR), and digital twins.
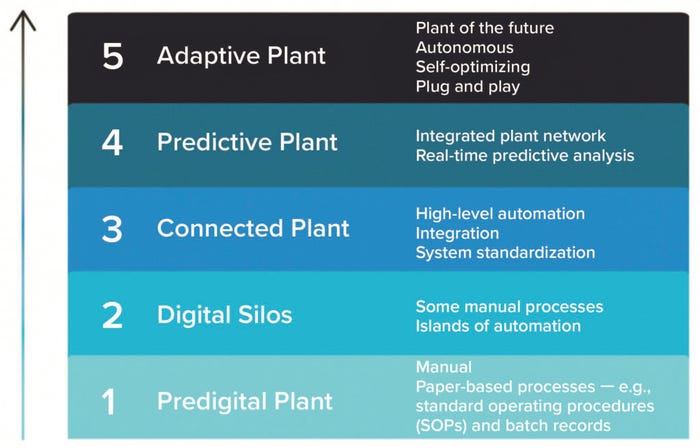
Figure 1: Digital plant maturity levels.
Digital Plant Maturity Model
The digital plant maturity model (DPMM) categorizes facilities based on their level of digitalization, automation, and data integration among teams (1). Figure 1 outlines the stages of evolution from a Level 1 to Level 5 facility.
Level 1 plants are predigital and still use manual, paper-based processes. Other characteristics of a Level 1 plant include low levels of automation, basic programmable logic controller (PLC) functions, and standalone applications with little to no handshaking.
Level 2 plants have slightly higher levels of automation mixed with manual processes. However, each piece of equipment is autonomous and does not share data with other equipment, creating digital silos and islands of automation. At this level, batch records are semielectronic or “paper on glass,” which entails scanning existing paper documents and creating digital versions.
Level 3 plants have vertical and horizontal integration of IT capabilities and a high level of automation. They use integrated enterprise resource planning (ERP), laboratory execution systems (LES), manufacturing execution systems (MES), and automation layers to support digitized business processes. Other characteristics include full electronic batch records, semiautomated analytics, and a standard application platform that’s adopted across the plant network.
Level 4 plants use integrated real-time process analytics, allowing manufacturers to predict issues and reduce downtime. Such facilities may have implemented advanced production technologies from the start and feature internal integration from plant to value chain, end-to-end supply chain visibility, and product development and manufacturing integration.
Level 5 plants are fully automated and self-optimizing. Although no manufacturing facility has technically reached this level yet, the goal is to design an adaptive plant with end-to-end value chain integration from suppliers to patients. Because everything would be plug-and-play, and quality theoretically is checked along the way, no downtime occurs.
Our 2021 Horizons: Life Sciences report indicates that slightly more than half of the 502 respondents rated their facilities at Level 3 (2). The responses also show that Big Tech and Biotech are further along in their digital journeys: The majority of start-up pharmaceutical and biotechnology CMO/CDMO/CRO respondents rated their facilities at Level 3 and below, whereas the majority of large bio/technology respondents rated themselves Level 4 (61%) and Level 5 (9%). Regardless of current maturity model level, nearly all respondents said that they want to achieve the next highest level within the next three years.
How do they get there?
Addressing the Challenges: Digital Tools
Because of the rigorous regulatory environment in which they operate, many companies within the biopharmaceutical industry hesitate to embrace digital tools. Furthermore, our report found that the top barriers respondents face in reaching their next DPMM level are related to cost, risk management, security concerns, and skill sets. However, reaching the next DPMM level is an attainable goal despite the perceived barriers.
Developing a phased or scalable approach that aligns digital tools with a manufacturer’s business goals and strategies addresses the challenges and barriers to implementation. In particular, introducing one tool at a time reduces stresses related to cost, culture, and processes. After all, introducing any new digital technology disrupts the status quo. Therefore, it becomes essential to ensure that an entire company supports adoption of such new technologies — all the way from the board room to the plant floor.
Reaching the Next DPMM Level: What Tools to Choose?
Companies use a plethora of digital technologies in their manufacturing processes to reach toward a Pharma 4.0 status and simultaneously move to the next DPMM level. Here are a few prevalent tools that I find to be particularly exciting.
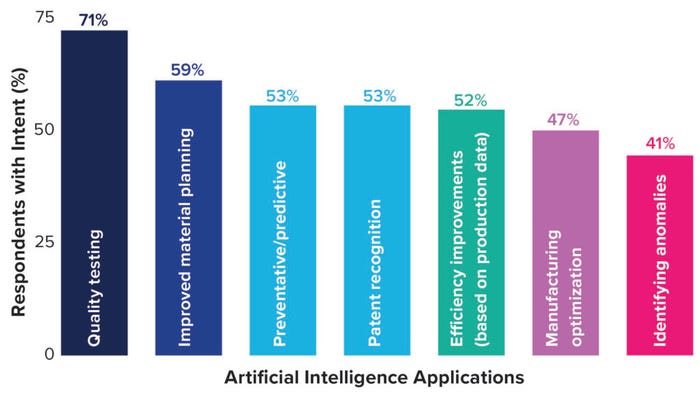
Figure 2: Artificial intelligence preferred application.
AI refers to information gathered and analyzed by a machine rather than a human. AI commonly is used in the biopharmaceutical industry to collect and analyze production data to improve efficiency and optimize manufacturing operations. Many companies use AI for predictive maintenance and analytics to anticipate failures and minimize production downtime. Respondents to our life-science survey stated that they intend to use AI for quality testing (71%), for improving material planning (59%), for predictive analytics (53%), and for improving efficiency (52%) (Figure 2).
Using AI enables the real-time analysis of feedback loops, thus allowing companies to make adjustments to improve their manufacturing processes. If your company is interested in quality by design (QbD), a robust AI system allows for quality testing using near–real-time, in-line quality control. Instead of waiting until the end of a manufacturing run to check product quality, connected AI systems check quality along the way and self-adjust.
Remote Manufacturing Tools (AR and VR): The COVID-19 pandemic created an abrupt, mandatory shift to remote work for many people around the world. Two pieces of digital technology that demonstrated significant value during this shift were AR and VR.
When meeting in person was no longer advised, many biopharmaceutical companies used AR and VR for collaborative efforts such as training, designing sessions, and factory acceptance testing (FAT) for new equipment. AR and VR also enabled companies to conduct remote audits of suppliers and partners when travel restrictions prevented them from doing so in person.
Despite the lessening travel restrictions, I suspect that such technology is here to stay. For example, using AR for FAT is an excellent option if you’re in a time crunch or don’t want to send your entire team on a trip. AR is particularly helpful for site construction walkthroughs and remote troubleshooting. Through “see what I see” technology, teams can walk through a project site with other personnel or conduct remote troubleshooting sessions with experts living around the globe. Adding to its aforementioned benefits, the cost of AR is relatively low — making it an affordable tool for companies that may be worried about the cost of reaching the next DPMM level.
Digital Twins: Manufacturers use digital twin technology to minimize disruptions to operations significantly. In short, a digital twin is a virtual and interactive duplicate of a company’s process control system or its physical facility. Creating a software digital twin enables companies to test changes and updates before those go live on their existing control system. This process ensures that no unintended consequences come to fruition. A company also can reduce the time spent on qualification efforts by implementing
software FAT qualification on a digital twin of its software.
Using 360° cameras or laser scanning, a company can create a digital twin of its facility to streamline operations. For example, without
actually being at the facility, a team member could look at a digital twin, click on a specific piece of equipment, and access its tag name, procedures, and cut sheets. Having that information at your fingertips no matter where you are creates many opportunities for optimization and efficiency.
Construction 4.0 Tools
Like Pharma 4.0 technologies, the Construction 4.0 concept does not describe a single digital tool, but rather a combination of digital tools working together toward a common goal. Biopharmaceutical companies implementing the aforementioned digital tools move toward realizing not only Pharma 4.0 status, but also a Construction 4.0 environment. Incorporating all the digitalization efforts applied at different stages throughout the manufacturing industry, Construction 4.0 translates those into a built environment, such as a biopharmaceutical manufacturing facility. With COVID-19 acting as a catalyst for implementing construction technologies and pushing them an estimated five years ahead of schedule, the Construction 4.0 concept is more relevant now than ever before.
Most advanced construction companies are at a DPMM Level 3, so they have made the transition away from data silos that commonly exist between design and construction teams. Job sites that leverage digital tools such as virtual reality, 3D modeling, and digital twins realize improvements in the speed and reliability of their design and construction cycles and benefit from enhanced communication between teams.
A Worthwhile Journey
The digital tools discussed herein — AI, AR, VR, and digital twins — just scratch the surface of diverse and complex Pharma 4.0 technologies. Although a large number of new and exciting technologies seem intimidating, implementing a scalable or phased approach to introducing digital tools helps move manufacturers to an increasing level of digital plant maturity. In the end, the rewards gained from achieving a fully automated and adaptive DPMM Level 5 plant will make the journey worth it.
Reference
1 Digital Plant Maturity Model. BioPhorum Operations Group, 2019; https://www.biophorum.com/resource/digital-plant-maturity-model.
2 Horizons: Life Science Reports. CRB 2021; https://go.crbgroup.com/horizons-life-sciences-report.
Yvonne Duckworth, PE, is director of digital technology at CRB; [email protected].
Pharma 4.0 is a trademark of the International Society for Pharmaceutical Engineering, Inc. (ISPE) (USPTO serial number #88234105). Construction 4.0 is a word trademark registered to BT & BT Management Consultancy Pvt. Ltd. at the Chennai, India, IP office.
You May Also Like





