Single-Use Processing for Microbial Fermentations
April 14, 2015
 During the past decade, single‑use bioprocessing has emerged as a standard platform for current good manufacturing practice (CGMP) mammalian cell culture. Biomanufacturers have come to appreciate the benefits of lower capital and operating costs, reduced contamination risk, continuity from early development through manufacturing, flexibility, and sustainability (1).
During the past decade, single‑use bioprocessing has emerged as a standard platform for current good manufacturing practice (CGMP) mammalian cell culture. Biomanufacturers have come to appreciate the benefits of lower capital and operating costs, reduced contamination risk, continuity from early development through manufacturing, flexibility, and sustainability (1).
Disposable cell‑culture vessels have gained wide acceptance because their performance duplicates that of stainless‑steel, fixed‑tank bioreactors, with which manufacturers have extensive experience. This is no accident: Single‑use bioreactors use stainless–steel engineering principles, particularly with respect to impeller rotation speed, agitation and aeration rate, reactor geometry, and so on. Retaining critical physical characteristics enables predictable, relatively problem‑free transitions from stainless to single‑use bioreactors.
Single‑use equipment is now widely used in mammalian bioprocesses outside of cell culture, including harvest, filtration, and buffer/media preparation. Disposable filtration and clarification are well established, and single‑use membrane adsorbers are beginning to replace polishing columns. But cost‑effective single‑use options for column chromatography media, cryopreservation, and process monitoring still prove elusive.
Fermentation
Whereas single‑use cell culture processes are now the norm, their acceptance in microbial fermentations has been rare (2). Fermentations are characterized by relatively large volumes, high titers, and relatively low production costs. Fermentation is principally the domain of nonglycosylated recombinant proteins and peptides such as insulin, erythropoietin, and interferon. But recently, several innovative, high‑value protein therapeutics manufactured through fermentation have entered development pipelines. Among these are antibody fragments, fusion proteins, nonglycosylated antibodies.
For such molecules, biomanufacturers face many of the same challenges they encountered with products made in mammalian culture during the early 2000s: namely regulatory risk and a dearth of reliable platform processes that reduce development costs and shorten time to market. Single‑use bioreactors have played a significant role in enabling those developments for cell‑culture processes. Thermo Fisher Scientific believes that the emergence of high‑value, microbially derived therapeutics will fundamentally and favorably change the economics of single‑use processing (3). Consequently, single‑use fermentors (SUFs) should become feasible — but only only if vendors make a clean break from current single‑use paradigms.
Single‑use cell‑culture systems are characterized by low power input and mixing capability, limited oxygen transfer, restrictive exhaust capacity, and poor foam management (4). Attempts to retrofit standard single‑use bioreactors to fermentations easily reveal those weaknesses. Within the current single‑use paradigm, conventional plastic bioreactors are suitable for perhaps the least challenging five percent of fermentations, thereby excluding an overwhelming number of current commercial processes.
In a couple examples of the performance of bioreactors sold for both cell culture and fermentation, a published application note for an Escherichia coli fermentation system (Cellution Biotech’s Celltainer product) reported that the system achieved a maximum OD600 of only 55.6 at five hours (5). A typical OD600 value for a fixed‑tank fermentor is about three times higher. Arguably the most ambitious work toward single‑use fermentation is the Xcellerex XDR‑50 MO fermentor system from GE Healthcare, which reports growth performance comparable with that of conventional stainless‑steel fermentors. But the maximum cell density for this 30‑L single‑use system is barely double that the Celltainer system (6). Additionally, Eppendorf and TAP Biosystems (now Sartorius Stedim Biotech) have introduced single‑use miniature‑size reactors that are suitable for fermentation process development and scale‑down experiments, but those technologies are not yet scalable.
Those examples demonstrate that retrofitting conventional single‑use bioreactors is insufficient for fermentations. Designing a true single‑use fermentor that duplicates performance of clean‑in‑place (CIP) vessels requires returning to engineering fundamentals and building something from the ground up.
Fermentation Fundamentals
Mass transfer drives fermentor design, particularly the ability to deliver oxygen to cells as measured by oxygen transfer rate (OTR). A second factor is heat transfer rate, the ability to remove heat.
Because oxygen is sparingly soluble in water, its concentration is often growth‑ limiting to fermentations. In typical microbial fermentations, the critical oxygen concentration is between 10% and 50% of saturated dissolved oxygen concentration (DO).OTR is a function of kLa (the volumetric mass transfer coefficient) and oxygen concentration relative to saturation conditions. The kLa value in turn is inversely proportional to bioreactor volume (which affects the rate at which gas may be introduced) and proportional to impeller RPM and the power input (Pg).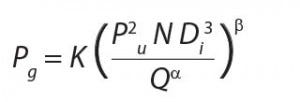
Pg is inversely proportional to aeration rate Q and proportional to impeller diameter D, the number of revolutions per minute N, and a coefficient K, which is related to reactor geometry. The equation below illustrates these points.
From that equation, it follows that a bioreactor design would not support fermentation because geometry, impeller types, energy input, and sparging differ significantly in the two systems.
Table 1 lists factors that can positively influence OTR. Reducing bubble size for a given oxygen flow rate or increasing gas flow rate improves transfer area but promotes foaming, and increasing bubble size results in low residence time. Faster impeller speed raises power requirements and can cause shear damage, and supplementing oxygen concentration in sparge gas can be costly.

Table 1: Finding the right balance between energy and cost efficiency while maintaining performance
The goal is to optimize performance by maintaining equilibrium between energy and cost efficiency (Figure 1). Achieving a proper balance requires maintaining kLa, agitation, and gas flow rate within modest limits as defined roughly by the enclosed area in the upper‑right segment of the figure. Extreme conditions lead to foaming, high cost, shear damage, oxygen limitations, poor mixing, or some combination of those factors.
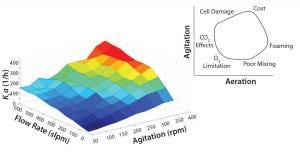
Figure 1: Achieving balance between agitation and aeration
Designing a Single-Use Fermentor
In designing a single‑use fermentor, Thermo Fisher Scientific returned to engineering principles applicable to microbial fermentation. The goal was to design a system that could be maintained within the boundaries shown in Figure 2.
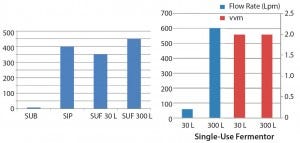
Figure 2: kLa performance (left) and airflow (right); vvm = vessel volumes per minute, Lpm = liters per minute
Fermentor characteristics should foremost duplicate the physical capabilities of stainless‑steel fermentors. The vessel should have an aspect ratio of 3:1 and a high turn‑down ratio to allow scaling (e.g., cell expansion or seed trains) within a single vessel. A 5:1 turndown ratio enables the reactor to operate at 20% nominal volume with efficient mixing. As with CIP systems, water‑ jacketed cooling ensures that heat generated during fermentation can be removed efficiently. Vessels should incorporate a high‑flow exhaust system that is capable of venting two vessel volumes per minute (vvm). Finally, inclusion of sensors for foaming, dissolved oxygen, pH, temperature, and other process attributes provides access to process analytical technologies (PATs).
Such design considerations support a drive train that is typical of fermentor vessels (e.g., three Rushton impellers to reduce bubble size, improve mixing distribution, and increase gas residency time). Impeller spacing — determined experimentally by spacing studies — is critical to achieving this performance level with no dead spots. Mixing power when properly baffled (up to 600 RPM and roughly 100‑fold higher than for bioreactors) should be sufficient to support fermentations while providing a means to break up vortexes.
The HyPerforma Fermentor
ThermoFisher Scientific introduced its HyPerforma single‑use fermentor system in October 2014. In designing the system, the company incorporated some features characteristic of fixed‑tank fermentors. Differences in aeration, sparging, and kLa between bioreactors and fermentors are striking. Air‑flow rates for a 30‑L cell culture single‑use bioreactor (SUB) amounts to <0.25 reactor volumes per minute, whereas values for a stainless‑stell fermentor and two HyPerforma reactors are comparable, with both values in the 2‑vvm range. Single‑use bioreactor kLa values invariably fall below 20/hour, whereas steam‑in‑place (SIP) fermentors can exceed 400/hour. Historically, measusring a kLa value >120/hour required compensation methods because of the inherently slow response time of conventional polarographic DO probes. New optical‑type DO sensors with greater than threefold faster response have shown the SUF delivering peak kLa exceeding 400 per/hour.
 It is no coincidence that biotechnology user groups such as DECHEMA in Germany have recently teamed up with PreSens Precision Sensing GmbH and key single‑use bioreactor manufactures in an effort to develop a standardized kLa protocol. Enhanced optical DO sensors and consistent analytical methods now allow end users to confidently compare performance among fermentor systems. Such studies show the Hyperforma SUF systems delivering kLa values exceeding 600/hour, leaving little doubt that single‑use technologies can now support microbial fermentation.
It is no coincidence that biotechnology user groups such as DECHEMA in Germany have recently teamed up with PreSens Precision Sensing GmbH and key single‑use bioreactor manufactures in an effort to develop a standardized kLa protocol. Enhanced optical DO sensors and consistent analytical methods now allow end users to confidently compare performance among fermentor systems. Such studies show the Hyperforma SUF systems delivering kLa values exceeding 600/hour, leaving little doubt that single‑use technologies can now support microbial fermentation.
Properly managing the sterile filtration of humid off‑gas is best achieved through a condenser system. Exhaust in legacy single‑use systems is restricted by the one‑inch or smaller tubing running from a SUB to a 3‑in. diameter filter capsule. Exhaust flow bottlenecks, and filter fouling can be major factors in bags failing and certainly contribute to operator anxiety.
A HyPerforma single‑use fermentor offers a recirculating exhaust condenser and uses a new, proprietary exhaust system in which the bag film is built onto and around the filter cartridge, thereby using the entire cartridge diameter for exhaust. Increasing the radius of the exhaust path from 0.5 in. to 1.5 in. provides a ninefold increase (π × 0.52 = 0.78 in.2 compared with π × 1.52 = 7.07 in.2), which is reflected in the greater than ninefold improvement in exhaust capacity.
Since the US Food and Drug Administration’s PAT initiative, manufacturers of both fixed‑tank and single‑use bioreactor designers have strived to provide systems capable of at‑line or in‑line analytics. The capacity for reading temperature, pressure, pH, and dissolved oxygen in real time is now considered standard for both fermentations and cell cultures. HyPerforma single‑use fermentors incorporate those analytics and relevant controls through industry‑standard probes and single‑use sensors. The fermentors also feature novel, proprietary, conductivity‑based single‑use foam sensors. Foam rising to the height of the probe completes an electrical circuit that triggers automated addition of antifoaming agents.
Real-World Examples
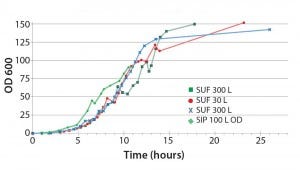
Figure 3: The 30- and 300-L single-use fermentor (SUF) performed comparable to the steam-in-place (SIP) 100-L fermentor; Escherichia coli BL21 DE3 cell density comparison (OD = optical density)
We tested 30‑ and 300‑L HyPerforma single‑use fermentors against an industry‑ standard New Brunswick Scientific BioFlo 100‑L stainless–steel fermentor for production of an 80‑kDa protein in E. coli. Figure 3 shows cell‑density measurements (by OD600 nm) for two 300‑L HyPerforma SUFs, a 30‑L HyPerforma SUF, and the BioFlo fermentor. Except for a brief early induction period in which the stainless‑steel fermentor outperformed HyPerforma, cell density in the four systems closely paralleled and were identical at 15 hours. We observed a similar pattern for dry cell weight (DCW). Performance may differ depending on culture conditions and product, but in our head‑to‑head comparisons, the HyPerforma SUFs values for OD600 nm and DCW, 146 and 47.4 g/L respectively, are squarely within the ranges expected for a good fixed‑tank fermentor.
We observed similar comparability for Pichia pastoris fermentations. In this case, a well‑defined small‑scale process from a Sartorius BIOSTAT DCU 7‑L stainless‑steel fermentor was scaled up and directly transferred into the SUF. Pichia are slower‑ growing than E. coli but generate more heat, which raises concerns about cooling capacity and foaming. Pichia also grow to significantly higher cell densities and dry cell weights, and their gas requirements are also higher: 3–4 vvm compared with 2‑vvm for E. coli (Figure 4). Our test showed exceptional agreement across all systems using the average of three runs each with two 30‑L HyPerforma SUFs, one 300‑L SUF, and a 7‑L SIP fermentor. OD600 nm (640) and DCW (91.7 g/L) values were squarely within the expected range for Pichia fermentations.
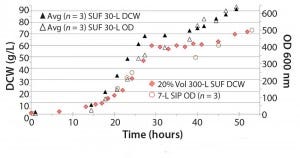
Figure 4: The 30 L and 300 L (at 20% volume) SUFs performed comparable to the SIP 7-L fermentor; Pichia pastoris GS115, 22 kDa protein; average comparison 640 OD600 nm, DCW 91.6 g/L (n =3); DCW= dry cell weight, OD = dry cell weight
Expanding Single-Use Technology to Fermenation
Mammalian‑cell culture has for years benefited from single‑use bioprocessing, but those advantages have until recently eluded microbial fermentation processes. This is no surprise to engineers familiar with the aeration, exhaust, cooling, and agitation requirements of both expression platforms. The differences are both quantitative and qualitative.
HyPerforma SUFs are the first dedicated single‑use fermentors based on well‑known engineering principles for bacterial and yeast fermentations. The economics of fermentations dictated that the scales initially introduced (30 L and 300 L) should cover development‑through‑industrial processes for high‑value fermentations. Industry end users indicate that the benefits of lower installation costs combined with the more rapid setup and deployment achieved by SUFs provide significant advantages for companies requiring rapid ramp‑up of bioprodution capacity while minimizing the risk of time‑ line and budget overruns. In the future we may find, as we did with mammalian cell culture, that capabilities for single‑use fermentation technology will expand to more commodity‑type products and even larger scales.
References
1 Shukla A, et al. Single‑Use Disposable Technologies for Biopharmaceutical Manufacturing. Trends Biotech. 31(3) 2013: 147– 154.
2 Dreher T, et al. Microbial High‑Cell‑Density Fermentations in a Stirred Single‑Use Bioreactor. Adv. Biochem. Eng. Biotech. 138, 2013: 127–147.
3 Lau M. A Novel Bispecific Antibody Targeting Tumor Necrosis Factor α and ED‑B Fibronectin Effectively Inhibits the Progression of Established Collagen‑Induced Arthritis. J. Biotech. 186, 2014: 1–12.
4 Eisenkraetzer D. Upstream and Downstream Process Technology: Bioreactors for Animal Cell Culture, Animal Cell Biotechnology. Biologics Production. Hauser H, Wagner R, Eds. Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2015.
5 lifetechnologies.com/suf? (see application notes listed at the bottom)
6 Microbial Fermentation in Single-Use Xcellerex™ XDR-50 MO Fermentor System. GE Healthcare Life Sciences: Uppsala, Sweden, 2013; http://goo.gl/GczpgV.
Nephi Jones is R&D Manager, Advanced Technology for the Bioprocess Production Division at Thermo Fisher Scientific, Thermo Fisher Scientific, 1726 HyClone Drive, Logan, UT 84321; 1-435-792-8736; nephi.jones@ thermofisher.com.
You May Also Like





