Enabling Faster Workflows with Protein Purification Technologies: Improvements in Chromatography and Electrophoresis
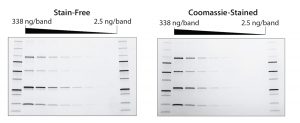
Figure 1: Stain-free gels are more sensitive than Coomassie-stained gels. TGX stain-free gels (BioRad Laboratories) were loaded with standards and a serial dilution of a protein mixture with four different proteins of varying tryptophan (Trp) content: phosphorylase B (PB, 1.4% Trp, 97 kD), glutamic dehydrogenase (GD, 0.8% Trp, 56 kD), carbonic anhydrase (CA, 2.3% Trp, 31 kD), and lysozyme (Lys, 3.4% Trp, 14 kD). Gels were run for 20 min at 300 V and imaged using a stain-free enabled imager (left). Gels were then stained with Coomassie stain and imaged using the same imager (right).
Purification of recombinant proteins is a critical step during protein therapeutics development. Protein therapeutics have a number of classifications based on their potential applications, including use as vaccines and diagnostics as well as for enzymatic, regulatory, or targeting activities (1). For all such applications, identifying and verifying protein purity is most important. Whether proteins themselves are therapeutics or the target proteins of interest, effective purification is essential in drug development.
Since the introduction of recombinant proteins in the early 1980s, use of protein therapeutics has expanded and become particularly pervasive throughout the fields of medicine and biotechnology. This increase is a result of their success as effective therapies and their extraordinary potential. The global market for protein therapeutics reached US$174.7 billion in 2015, with the US market at nearly $92 billion (2). More than 60 protein therapies have been approved by the US Food and Drug Administration: About 140 are in clinical trials, and more than 500 are in preclinical development (3). The effectiveness and prominence of recombinant proteins as therapeutics necessitates thorough and accurate formulation and development, which require fast and cost-effective methods of protein purification.
Proteins are produced by cell culture and are most often are isolated from those host cells using chromatography. Automated chromatography can be used to accelerate existing workflows by speeding up separation and isolation of proteins with minimal hands-on intervention. The technology improves sample purity and maintains yield. For proteins with similar elution times, however, chromatography alone cannot ensure sample purity. In such cases, an additional technique is required to confirm purity throughout a protein-purification workflow.
Electrophoresis is a preferred choice for protein characterization because it effectively separates proteins that are not resolved on a chromatogram and allows for further downstream protein characterization. But electrophoresis is a time-consuming procedure: Sample preparation, staining, destaining, and imaging can take a full day. Recent innovations in stain-free electrophoresis greatly reduce impact on characterization workflow. With new technology, laboratory professionals can run samples in <30 minutes.
Automated Chromatography for Protein Purification
As mentioned above, liquid chromatography remains the most popular method of recombinant protein purification and isolation (4). Recombinant proteins in therapeutics must be correctly folded and modified posttranslation. Glycosylation in particular is significant for recombinant proteins because its effect on antibody presentation is necessary for patients to tolerate drug products. Protein production in mammalian host cells can achieve such modifications. But their use also necessitates separation of therapeutic proteins from complex biological mixtures to eliminate carryover of host-cell residual material into final products and prevent adverse events.
Laboratory professionals have used affinity, ion-exchange, and other chromatography methods to improve sample purity without sacrificing yield. New chromatography technologies continue to build on an established three-decade best practice by introducing automation and customization. New software in chromatography systems allows workflow steps — and even complicated, multistep procedures — to be automated and monitored remotely. Reducing human involvement decreases the time and cost associated with chromatography and minimizes human error. Customization allows for increased throughput and sample purity because laboratory professionals tailor equipment settings to particular samples.
Although chromatography continues to become more effective at maximizing protein purity and sample yield, it is not always effective at differentiating proteins with similar elution times. A chromatogram peak can represent two proteins if they elute at similar times. As such, a chromatogram alone is not sufficient to determine sample purity.
Chromatography is useful for initial purification steps. Use of a secondary procedure (e.g., gel electrophoresis) allows for further separation to distinguish proteins with similar elution times and facilitates sample preparation for downstream characterization and structural analysis (5).
Accelerating Gel Electrophoresis Procedures
Gel electrophoresis is an orthogonal procedure for separating and subsequently identifying proteins through downstream assays such as Western blotting. When combined with chromatography, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) is an effective method of ensuring fraction and final sample purity throughout every stage of a purification process.
Standard operating procedures (SOPs) for protein therapeutics often require confirming purity at every stage of a purification workflow. Less-regulated processes can allow researchers to skip purification checks and infer fraction purity from previous purifications and other data (6). Highly regulated procedures — such as good manufacturing practice (GMP) laboratories — often require such assessments. However, the time-intensive nature of SDS-PAGE means that when the number of required purity assessments is increased substantially, so are both the time and costs associated with drug development.
The critical nature of protein therapeutics development requires that sample quality be of the utmost importance. As a result, procedures are highly regulated. It is not acceptable, therefore, to infer fraction purity before pooling fractions, which must be assessed at the end of a purification workflow and after each intermediary step. Similarly, it is important to ensure the purity of fractions without significantly compromising yield. Increasing the number of required assessments can ensure a sample’s high quality, but it can also reduce its size unless appropriate measures are taken.
Although SDS-PAGE can satisfy requirements for both sample purity and yield, the process can take a full day. When time and costs are critical concerns, researchers often cannot afford to spend a full day verifying protein quality after each step of a purification workflow that includes multiple columns. Technology has attempted to reduce the time required for electrophoresis by increasing the speed of gel runs and decreasing time required for staining. But the greatest time decrease occurs when staining is completely removed from a procedure. Stain-free technology can reduce run times to ≤30 minutes, thereby drastically cutting costs and time required to determine sample purity.
Stain-free electrophoresis technology uses ultraviolet (UV) irradiation to target aromatic amino acids in native proteins. When exposed to UV radiation, amino acid residues (commonly tryptophan) can be modified with trihalo compounds. Binding a 58-Da moiety increases the fluorescence of tryptophan under UV light, thereby allowing researchers to visualize a sample easily (7).
Notably, the moiety does not interfere with downstream analyses. So SDS-PAGE still can be used as the first step in protein characterization before Western blotting (8), structural analysis through circular dichroism (9), nuclear magnetic resonance (10), and activity analysis through surface plasmon resonance and enzyme-linked immunosorbent assay (11). In addition to its use in protein identification for sample purity, SDS-PAGE can function as a preliminary step in the analysis of multimeric protein complexes.
The rapid run times of stain-free procedures can be attributed to a lack of staining and destaining steps that are typically necessary for protein visualization. Stain-free gels can be used with the same buffers and reagents as standard SDS-PAGE gels. Stain-free procedures also provide increased dynamic range, increased sensitivity, and a lower limit of detection compared with standard Coomassie staining methods (Figure 1) (12, 13). Quality is not sacrificed for speed. Eliminating staining and destaining in favor of UV irradiation and fluorescence results in a more-effective procedure without the use of toxic and hazardous organic material.
Faster SDS-PAGE procedures due to stain-free technology allows them to be used as a complement to chromatography at every stage of a protein purification workflow. This in turn helps ensure sample purity, which is critical in drug discovery and development applications.
Figure 2: Following chromatography, drug discovery researchers can confirm the purity and yield of their proteins with stain-free gels in under 30 minutes.
Meeting Analytical Needs
An increasing number of protein therapeutics in the industry necessitates the development of faster, more cost effective, and generally more effective workflows (Figure 2). Greater competition in this area means that biomanufacturers need to decrease their time to market. The development of new technologies can help meet the demand for new drug products. As the market continues to grow, protein purification procedures must become viable for both small-scale drug discovery and process-scale applications.
Stain-free gel electrophoresis significantly improves SDS-PAGE procedures for validating protein purity. Because chromatography is not effective enough on its own to ensure protein purity for critical applications such as drug discovery, effective orthogonal procedures are necessary. Stain-free technology drastically reduces run times while increasing sensitivity, dynamic range, and the lower limit of detection. When coupled with advances to automated chromatography systems, protein purification workflows can be completed faster and at lower cost than ever before at both small and process scales.
References
1 Leader, Baca QJ, and Golan DE. Protein Therapeutics: A Summary and
Pharmacological Pharmacological Classification. Nat. Rev. Drug Discov. 7(1) 2008: 21–39.
2 Technology Advancements in Protein Therapeutics Spurring Market Growth, Reports BCC Research. Marketwired: Wellesley, MA, 25 May 2016.
3 Kaspar AA, Reichert JM. Future Directions for Peptide Therapeutics Development. Drug Discov. Today 18(17–18) 2013: 807–817; doi: 10.1016/j.drudis.2013.05.01.
4 Carr CD. The Role of Chromatography in the Characterization and Analysis of Protein Therapeutic Drugs. LCGC 32(4) 2014: S24–S29.
5 Garfin DE. One-Dimensional Gel Electrophoresis. Methods Enzymol. 182, 1990: 425–441.
6 Raynal B, et al. Quality Assessment and Optimization of Purified Protein Samples: Why and How? Microb. Cell Fact. 13, 2014: 180; doi: 10.1186/s12934-014-0180-6.
7 Yadav G, et al. Trends in Protein Separation and Analysis: The Advance of Stain-Free Technology. Bioradiations 9 Sep. 2014; www.bioradiations.com/trends-in-protein-separation-and-analysis-the-advance-of-stain-free-technology.
8 Rivero-Gutierrez B, et al. Stain-Free Detection As Loading Control Alternative to Ponceau and Housekeeping Protein Immunodetection in Western Blotting. Anal. Biochem. 467, 2014: 1–3.
9 Johnson Jr WC. Protein Secondary Structure and Circular Dichroism: A Practical Guide. Proteins 7(3) 1990: 205–214.
10 Cavanagh J, et al. Protein NMR Spectroscopy: Principles and Practice, 2nd ed. Elsevier Academic Press: Burlington, MA, 2007.
11 Karlsson R. SPR for Molecular Interaction Analysis: A Review of Emerging Application Areas. J. Mol. Recognit. 17(3) 2004: 151–161.
12 Gilda JE and Gomes AV. Stain-Free Total Protein Staining Is a Superior Loading Control to β-actin for Western Blots. Anal. Biochem. 440(2) 2013: 186–188.
13 Taylor SC, et al. A Defined Methodology for Reliable Quantification of Western Blot Data. Mol. Biotechnol. 55(3) 2013: 217–226.
Corresponding author Laura Moriarty, PhD, is drug discovery and development business marketing manager, and Gunjan Choudhary, PhD, is technical writer/editor at Bio-Rad Laboratories, 6000 James Watson Hercules, CA 94547; [email protected].
You May Also Like





