- Sponsored Content
- Chromatography
Improving the Performance of Tried-and-True Chromatography Technology
March 16, 2022
Sponsored by Astrea Bioseparations
Efficient and effective downstream processing of biopharmaceuticals reduces manufacturing costs and time. Chromatography is the primary purification method for traditional recombinant proteins, monoclonal antibodies (MAbs), plasmid DNA, and viral vectors. Although interest in membrane separation technologies is growing, traditional resin-based solutions continue to be preferred when high-resolution purification is required.
Ion-exchange chromatography (IEC)(e.g., cation and anion-exchange chemistries) and hydrophobic-interaction chromatography (HIC) are established bioseparation technologies. Cation-exchange resins are used widely for MAb polishing and aggregate clearance steps. Anion-exchange (AEX) resins are used for polishing viral vectors based on adenoassociated viruses (AAVs). Most plasmid DNA produced to support the manufacture of mRNA and viral vector vaccines and gene and gene-modified cell therapies are purified using HIC resins during a polishing step.
Over the past several decades, resin-based chromatography has proven to be a tried-and-true technology in the biopharmaceutical industry. It continues to be used in the downstream processing of both conventional and next-generation biologics.
Improved Performance at the Resin-Bead Level
Often, resin beads are manufactured using processes that do not enable precise control of bead size. Many bead-formation processes use organic solvents, which can produce beads of different sizes. That heterogeneity influences flow through a resin bed and the potential for the presence of residual solvents. It also has negative environmental impacts.
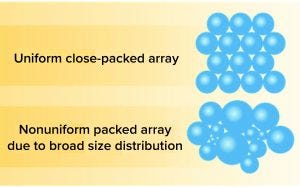
Figure 1: Monodispersity of PuraBead 6HF base matrix for the production of ion-exchange and hydrophobic-interaction chromatography resins.
Astrea Bioseparations has developed a highly controlled, aqueous bead-manufacturing process. The PuraBead 6HF base matrix (6%) agarose resin beads produced in this process are highly uniform (Figure 1), thus enabling excellent flow rates and low back pressures. The near-monodisperse particle-size distribution of the PuraBead 6HF base matrix provides high resolution and good binding properties, reduces the risk of column fouling, and renders these adsorbents easy to pack (Figure 2). Because no organic solvents are used in bead production, the process does not generate solvent residue.
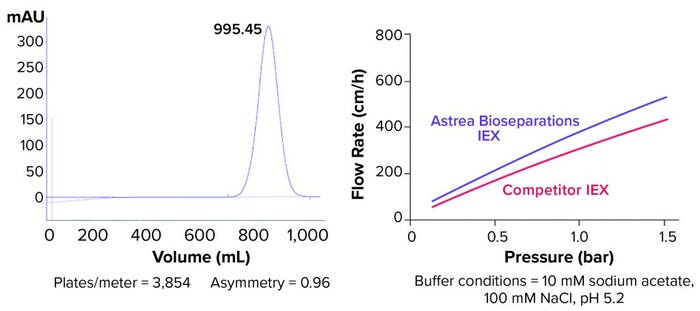
Figure 2: (left) Typical packing study for Astrea Bioseparations’s PuraBead HF IEX adsorbents (10-cm–diameter column, ~14-cm bed height) shows asymmetry results in the range of 0.8 to 1.2. (right) Flow rates for the PuraBead HF IEX adsorbent and a competitor agarose IEX resin (10-cm–diameter column, 20-cm bed height) are compared.
The matrix of the base beads is highly crosslinked to provide a material that also supports fast flow rates. The crosslinked beads are further derivatized with different well-known synthetic ligands to generate IEC (cationic or anionic) and HIC chromatography resins.
Both weak and strong PuraBead HF anion- and cation-exchange adsorbents are available, including diethylaminoethyl (DEAE), quaternary ammonium (Q), carboxymethyl (CM), and sulfonic acid with a propyl linker (SP). Astrea Bioseparations also offers PuraBead HF HIC resins with butyl, hexyl and octyl, and phenyl ligands.
PuraBead HF resins offer excellent performance for demanding process applications, including capture, intermediate purification, and polishing steps. The resins are designed to be stable to 1 M NaOH for sanitization and clean-in-place operations. One example demonstrates retention of high performance for the SP PuraBead HF resin over 200 cycles with cleaning using 0.5 M NaOH every cycle.
Secured Resin Supply
Higher performance of chromatography resins can be achieved only if the supply of those resins is reliable. With the current escalating supply-chain issues amid a rapidly growing biopharmaceutical market, biomanufacturers must identify raw-material suppliers that can offer assurance of supply. Production of resin beads at the Astrea Bioseparations facility in the United Kingdom shortens the supply chain for chromatographic media. Thus, the company generates a highly consistent, high-performing product with minimal supply-chain risks — increasing the assurance of supply for its customers.
Astrea Bioseparations has a long-proven track record of supplying high-quality chromatographic media that meet and exceed the quality and regulatory requirements for supporting bioprocesses from clinical to good manufacturing practice (GMP) phases. Twenty-one of the company’s resins are used for the purification of FDA/EMA-approved products, and many more candidates are progressing through clinical trials.
Although many IEX and HIC ligand chemistries are generic, considerable variations in ligand-attachment chemistries, ligand densities, and base-bead characteristics could affect overall resin performance. If process improvements are required, screening with alternative resins such as PuraBead HF IEX and HIC resins is highly recommended. Performance based on ligand chemistry alone does not provide the whole picture.
Greater Process Efficiency
Chromatography resins and unique bead technologies can be purchased for packing in a column of your choice or in one of the Astrea Bioseparations’s plug-and-play, prepacked 1-mL and 5-mL columns for research applications using IEC or HIC resins. IEC resins also are available for research and development through to GMP manufacturing at pilot and commercial scales (volumes ≤6 L).
Prepacked columns with nonmetallic flow paths eliminate the need for lengthy column preparation, packing, qualification, and sanitization, thus streamlining workflows while saving time and money. EvolveD process columns are packed in a GMP (ISO 7) environment and supplied with a certificate of analysis and regulatory support file (RSF).
Available kits containing research-scale prepacked columns can be used to evaluate the performance of different resin types. Screening kits feature columns prepacked with each of the four different IEC and HIC resins to help determine which type of resin is best for each process. Once the optimal resin is identified, a kit can be purchased containing four research-scale columns prepacked with that resin for parallel screening of process conditions. Astrea Bioseparations is working to produce IEX and HIC prepacked columns in a universal format suitable for high-throughput liquid handling, with launch expected in 2022.
A Foundation of Support
Investing in the development of high-throughput solutions is just one way that Astrea Bioseparations is expanding support for customers tackling problematic chromatography processes. The company is in growth mode and focused on innovating solutions for customer needs. Critical infrastructure investments continue to support innovation, including an application development laboratory to focus on viral vector manufacturing for gene therapies. Once that is completed, the company can produce gene therapy vectors for developing fit-for-purpose purification media and platforms.
The company also has been working on developing an efficient and cost-effective generic capture chromatographic process for AAV vectors, as an alternative to affinity chromatography. Methods of polishing AAV and plasmid DNA targets using HIC and ion exchange are also under development. Application notes will be prepared and shared to provide deeper insights at each development stage.
Astrea Bioseparations’s applications research and development can cover a multiple challenges in the industry thanks to the coordinated relationships across a group of complementary companies developing advanced manufacturing solutions and delivering access to information and connectivity. A workflow-solutions approach is applied, empowered by access to enabling equipment and consumables portfolios of Univercells Technologies and Mirus Bio for upstream processing.
Work also is being done to address the limitations and difficulties associated with using traditional chromatography resins for large-molecule modalities such as cell and gene therapy. For both IEC and HIC, ligands are linked to the resin beads that interact through different mechanisms with target drug substances. The characteristics of those resin beads can influence the performance of fully formulated resins significantly. Novel chromatographic media that combine proven workhorse ligands with advanced matrices can increase efficiency and separation performance without the need to make major process changes.
In 2022, Astrea Bioseparations is positioned to bring transformative bioprocessing solutions to drug developers. The company will continue leveraging its decades of expertise in ligand and ligand-attachment chemistries and advanced resin-bead solutions with new technologies to solve the complex downstream purification challenges of today’s developers.
Ian Scanlon is a subject matter expert at Astrea Bioseparations, a part of Gamma Biosciences; [email protected]
You May Also Like





