- Sponsored Content
- Chromatography
Prepacked Chromatography Columns for Risk Reduction: Statistical Process-Control Analysis Demonstrates Performance Consistency
November 16, 2023
Sponsored Content
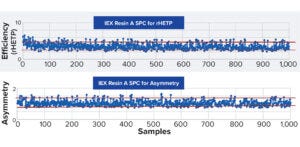
Figure 1: More than 1,000 data points demonstrate reproducible ion-exchange (IEX) resin packing while maintaining robust quality across eight years. Red lines denote upper and lower confidence limits, with 99% <3σ and 96% <2σ for efficiency, and 98% <2σ and 99% <3σ for asymmetry. rHETP = reduced height equivalent of theoretical plate.
In September 2023, Adam Nelson (chromatography product manager at Repligen Corporation) led an Ask the Expert webinar on applying statistical process-control (SPC) analysis to assess performance consistency for prepacked chromatography columns.
Nelson’s Presentation
OPUS prepacked chromatography columns come in a range of formats, from small-scale RoboColumn, MiniChrom, and ValiChrom columns for process development and process validation to large scale columns for clinical and commercial manufacturing. Customers specify the diameter, bed height, and resin to be packed inside their columns. As the product line has expanded from its initial launch in 2012, Repligen has gathered thousands of columns’ worth of data — using that information for continuous improvement.
Nelson showed a trending graph for asymmetry and efficiency from thousands of columns with more than 250 resin types. Using SPC, the OPUS team can share data with customers for improved understanding of process controls and how they relate to column performance. Results show how column size, bed height, and diameter can optimize a process.
A significant data set from years of packing columns has gone into process-control charting software and methods that leverage data variance and standard deviations to determine control scores. Repligen teams use those results to evaluate and optimize packing methods in a plan–do–act–check–repeat cycle.
Nelson shared details of an ion-exchange resin family in different column diameters and bed heights, with data from thousands of individually packed columns. To determine upper and lower control limits, efficiency is expressed as reduced height equivalent to a theoretical plate (HETP). Over 95% of the data fell within the standard-deviation, giving >95% that a customer’s individually tested column will conform to the hundreds of applicable data points in the OPUS column. For asymmetry, the trend was similar, with >97% of results falling within 2σ of the standard deviation. Such results give users confidence that they will receive consistently packed columns that will perform reliably.
The data set can be filtered by parameters such as column diameter, which helps users assess scalability of OPUS columns. Refining results by resin type — for example, an affinity resin tested at a certain column diameter and bed height — further tightens the distribution of data. Repligen standard specifications are used for testing columns, providing consistency in processes and column sizing, and reproducibility for scaling. Sample data for a 45 cm × 25 cm column was tightly distributed even with relatively few data points. SPC for an adenoassociated virus (AAV) affinity resin showed >98% of results falling within 3σ for efficiency and 99% for asymmetry (Figure 1). Using OPUS columns minimizes variability in packing columns, helping to optimize project timelines. Standard specifications for packing and testing translate into consistency of performance.
More Online
Watch the recorded presentation online for more detailed discussion, including slides and a question-and-answer session: https://bioprocessintl.com/sponsored-content/pre-packed-chromatography-columns-for-risk-reduction-statistical-process-control-analysis-demonstrates-performance-consistency.
Find the full webinar online at www.bioprocessintl.com/category/webinars.
You May Also Like





