- Sponsored Content
- Downstream Processing
Why, Why, Why… ELISA? A Look at the Benchmark HCP Assay
May 25, 2020
Sponsored by Cytiva
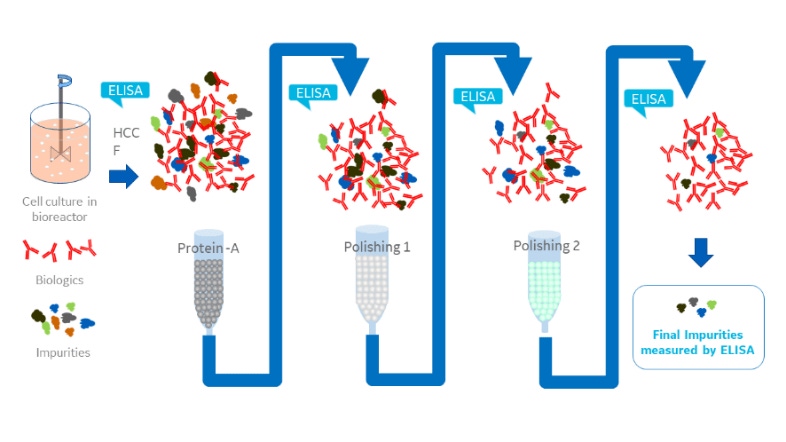
Host cell proteins (HCPs) are a primary source of impurity in biologics manufacturing. When present in drug formulations, HCPs can reduce efficacy, introduce toxicity, and increase risk of long-term immunogenicity. Understanding HCP profiles and integrating effective removal strategies are critical when developing a new biological drug, both for ensuring patient safety and fulfilling regulatory guidelines.
HCP populations can be complex and structurally diverse, and most changes in upstream culture conditions affect HCP concentrations and control strategies. Accurate and reliable HCP quantitation is essential to check the effect of each adjustment, optimize purification steps, and ensure adequate removal.
In this white paper, we explain why and how analytical scientists use the ELISA to perform effective HCP quantitation throughout process development. We also discuss how biopharmaceutical companies modify sandwich ELISA protocols to employ multiple polyclonal antibodies against complex HCP populations in a single multi-analyte assay.
About the Author(s)
You May Also Like





