Change continues to abound in BPI’s world, most recently with the launch of our newly redesigned and mobile-optimized website at
https://www.bioprocessintl.com
. It should look familiar to you but refreshed, and we editors are most pleased with the refined search function. That should make it easier for you to find what you’re looking for in our ever-expanding content archive. The new site is a little faster and better integrated with the BPI events sites now as well.
To download PDFs of our articles — which are often the best presentations for our technical content — you will have to reregister for the site. We have a new platform and service provider running things, and the registration data did not transfer directly from the old host. If you’re looking for a digital page-turner, you can find issues at
https://micro.zsites.co/view/lite/5cd1dc00faf7ea4183ac75d3
going back to 2010. I find that format to be surprisingly easy to navigate and read on screen.
Also “new and improved” this year will be our ...
Pluripotent stem cell (PSC) therapies enable developers to address some of the most difficult diseases and injuries. Accordingly, interest in such therapies is growing rapidly. The number of PSC-therapy clinical trials conducted around the world increased from 12 in 2015 to more than 90 in 2020 (
1
). A Mordor Intelligence study suggests that, by 2028, the induced pluripotent stem cell (iPSC) therapy market could be worth ~US$2 billion — up sharply from 2023’s ~$1.2 billion valuation (
2
).
Despite growing interest from drug companies, traditional approaches to PSC development often rely on short-term strategies that are replete with risks that hinder many promising therapies from reaching the market. Unaddressed risks in PSC-therapy development can have downstream consequences that threaten even the most promising programs. Scientists need a new developmental framework to realize the potential of PSC therapies.
Planning for Success
Sourcing Suitable Cell Lines:
Challenges with PSC development can begin ...
The advent of advanced therapy medicinal products (ATMPs) has ushered in a new age of healthcare by providing curative solutions to some of the world’s most debilitating diseases. ATMPs represent an inflection point in our industry’s ability to improve patient treatment. A November 2023 report states that 34,400 patients were treated with chimeric antigen receptor (CAR)-T cell therapy, which is being hailed as the “holy grail” of cancer therapies because, in some cases, it can achieve complete response (CR) rates of >80%. (
1, 2
). Drug developers reached this milestone thanks to decades of research that broadened our understanding of the underlying biology, immunology, and genetic engineering that drive new therapies toward favorable clinical outcomes.
But the ATMP revolution in medicine presents a unique set of challenges, especially regarding advanced manufacturing, logistics, storage, and data analysis. Groundbreaking manufacturing and digital technologies are now integral to ATMP development. Such co...
The biopharmaceutical industry has reached an inflection point regarding chimeric antigen receptor (CAR) technology. CAR-bearing T cells have become an established treatment modality for hematological indications. Consider that before such products emerged in clinical and commercial contexts, children and young adults with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL) expected five-year survival rates of 21% and 10%, respectively. Today, 70–90% of B-ALL patients who are treated with CD19-directed CAR T cells can achieve complete remission (
1
). Since the 2017 debut of Kymriah (tisagenlecleucel) from Novartis, five other such products have received US Food and Drug Administration (FDA) approval (
2
). CAR-T has become a second-line treatment option in some cases (
3
). Drug developers recognize, however, that the modality continues to have limited efficacy against solid tumors, which represent 90% of cancers in human adults and 30% of those in children (
4–6
). Thus, CAR-based approa...
Cardiovascular diseases represent the leading cause of death globally, with nearly 18 million fatalities occurring each year (
1
). Pharmacological interventions mainly address symptoms rather than root causes of such diseases. Unfortunately, many patients do not respond adequately to treatment, so their conditions progress to congestive heart failure, eventually necessitating heart transplant. Organ supplies are limited, however, with a waiting list that continues to grow each year. Complications also can arise from immunosuppressive drugs that patients must take indefinitely after receiving donor hearts, significantly limiting applications of the transplant option.
Cell-based therapies have emerged as a promising alternative because they could regenerate heart tissue. Results from several clinical studies — based on different experimental designs and involving more than 3,000 patients in total — confirm the feasibility and safety of such procedures. Conducting one of those studies was Philippe Hénon, th...
Regenerative medicine is ushering in a paradigm shift focused on therapeutic cell delivery for personalized medicine. Mesenchymal stromal cells (MSCs) have garnered extensive attention from both academic and industrial sectors since their discovery over 50 years ago. The unique properties of MSCs, including their multilineage differentiation and immunomodulatory effects, have propelled research into the pursuit of therapeutic applications. To date, 474 clinical trials have been completed using MSCs to treat an array of diseases, such as acute myocardial infarction, knee osteoarthritis, rheumatoid arthritis, and diabetes (
1–4
).
Despite the widespread interest in MSCs, they are scarce in human bodies, constituting just 0.01% and 0.005% of the bone-marrow mononucleated cell (BMMNC) population for males and females, respectively (
5
). With such low cell counts, large volumes of bone-marrow aspirate (BMA) and extended culture times are needed for sufficient MSC expansion in downstream processing and therape...
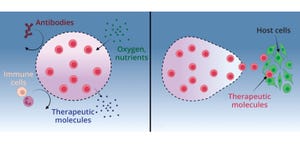
Although stem-cell therapies (SCTs) hold significant promise for treating chronic disease, a major difficulty in their clinical use comes with the high levels of cell death and low levels of cell retention in target locations after transplantation. Intravenous (IV) injection is a common and minimally invasive method of delivery for cell transplants (
1
). It may seem to be an ideal choice to provide large doses of cells, but IV transplantation is limited by insufficient homing, which leads to transplanted cells either dying without leaving circulation or becoming trapped in undesired organs (
2, 3
).
One way to address problems with viability and retention is to use supratherapeutic doses or repeated injections to ensure that sufficient cell numbers survive and reach their target region (
4–6
). However, high doses and serial injections not only exacerbate the issues of cost and scalability, but also introduce significant safety concerns (
7
).
Several biomolecular approaches to addressing transplanted ce...
Sponsored Content
Rev up your AAV genome production in upstream process development and manufacturing
Designed to deliver a higher AAV titer for:
Mirus Bio has developed the RevIT AAV Enhancer to further increase AAV genome titers in suspension 293-derived cells.
This novel enhancer is easily integrated into existing workflows and produces 2-4X* higher titers across a range of serotypes, cellular growth media formulations, and transfection platforms.
The RevIT AAV Enhancer can further drive down upstream manufacturing costs by lowering levels of plasmid DNA while maintaining high-quality titers.
Fill out the form below to read the full article now.
Sponsored Content
In 2023, the biopharmaceutical industry marked a record year with 71 new drug approvals (
1
). Seven of those were cell and gene therapies (CGTs), including the first CRISPR/Cas9-edited therapies, bringing the total of CGTs approved by the US Food and Drug Administration (FDA) to 34. The thousands of other CGT candidates in preclinical development highlight the rapid growth and promising future of these modalities.
Clinical success across a growing list of conditions has placed significant demands on CGT developers to speed therapies to the market. The high cost per dose has added pressure to reduce cost of goods and increase patient access, necessitating high-yielding, productive, and efficient production processes. A 2023 survey also reports that although 90% of respondents held a positive outlook for the CGT industry over the next 12–18 months, nearly 40% lacked confidence in their current manufacturing capabilities (
2
).
CGT clinical trials account for <2% of all trials listed with US regulators but ...
With 35 years of experience in biomanufacturing, Richter-Helm BioLogics provides best-in-class solutions as a contract development and manufacturing organization (CDMO). We offer high-quality services from process and analytical method development to commercial manufacturing that is compliant with current good manufacturing practices (CGMPs). Such work requires expertise in process and analytical validation and process performance qualification (PPQ) to produce therapeutic proteins, peptides, antibody-like scaffolds, bacterial vaccines, and plasmid DNA (pDNA). Richter-Helm’s services are inspected routinely by regulatory authorities, such as the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA), to maintain high quality standards.
What sets us apart from other CDMOs is a strong customer focus. We think of our customers as partners, and we take their issues seriously. We give full attention to each product we manufacture to support our mission of providing high-level CDMO soluti...
Digital polymerase chain reaction (dPCR) technology is used increasingly for absolute quantification of nucleic acids in research, diagnostics, bioproduction, and environmental testing. In a September 2023 Ask the Expert webinar, Samyuktha Shankar (field application specialist for the Pharma Analytics group with Thermo Fisher Scientific) discussed leveraging dPCR for absolute quantification of lentiviral vector (LVV) samples. Shankar explained how the technique reduces variability and improves both accuracy and analytical sensitivity even when high-background conditions are present.
Shankar’s Presentation
Vector copy number (VCN) is a critical quality attribute (CQA) for assessing integration and safety of transduced cell-therapy products. Because LVVs are used to transduce cells, they are considered to be an active drug-substance ingredient. Regulatory guidelines require drug substances to be tested for identity, purity, potency, safety, and stability.
Once cells have been transduced, they must be tested...
Tangential-flow filtration (TFF) membrane performance is difficult to maintain between batches and during scale-up. Lucas Smith (product manager at Repligen) discussed the benefits of good mixing during TFF in a recent BPI Ask the Expert webinar. In laboratory-scale processes, recirculation flow into the vessel may provide adequate mixing. However, in large-scale tanks, issues such as inconsistent feeds and heterogeneity can cause poor membrane performance. Using a method for evaluating mixing efficiency via its impact on membrane performance, Repligen presented a study comparing traditional mixers with their Tulip Tank technology.
Smith’s Presentation
Many TFF vessels provide inadequate mixing, allowing material to stratify, localize, or short-circuit. Poorly mixed material enter the filter nearly untouched, creating a portion of feed with an abnormally high concentration that leads to compromised membrane performance. Drug manufacturers must adopt a method to analyze feed homogeneity during early stage ...
Although chimeric antigen receptor (CAR) T-cell cancer therapies have the potential to cure cancer, they rely on complex drug customization for each patient. CAR-T manufacturers have spent years trying to personalize manufacturing processes, but their strategies rely on technicians with extensive immunology and scientific experience. Companies have struggled to achieve success using that model.
Despite abundant venture investment, CAR-T developers often encounter delayed schedules and excessive expenditures while they try to refine complicated and difficult-to-scale manufacturing processes to generate clinical proof of concept. Prominent CAR-T companies recently announced high-profile staffing cuts and have narrowed the reach of their clinical programs.
Fortunately, CAR-T manufacturing is similar for all companies, differentiated mostly by how they genetically modify cells. Over 90% of production is virtually identical for each patient, which enables companies to use paradigm-shifting techniques that the ...