Luina Bio, one of Australia’s most experienced biopharmaceutical contract development and manufacturing organizations, has announced plans to open a new good manufacturing practice (GMP) manufacturing suite. The expansion plans include a new small-scale GMP manufacturing suite that is scheduled to open in the fourth quarter of 2020. The suite will complete the company’s service offerings to customers that require small-scale GMP facilities.
“This new suite will allow us to respond to those customers that need a smaller active dose for their initial clinical development but cannot easily find the right facility,” said Luina Bio CEO Les Tillack.
Fill out the form below to read the complete capabilities review and learn more about Luina Bio’s small-scale GMP manufacturing suite now
.
In all bioprocess stages, developers and manufacturers aim to intensify as many steps as possible. For upstream processes, the result can be significant productivity gains with an evolution of bioreactor train design that takes advantage of higher titers to compress the train and intensify production outputs. Upstream intensification of production-scale bioreactors has driven bioprocess productivity evolution for several years. Interest has been growing in what can be achieved with laboratory-scale cell expansion, which has remained a fairly static process involving serial scale-up from vials to multiple flask volumes. Those steps are performed before bioreactor scale for production and before additional process development and characterization at another laboratory.
Fill out the form below to read the complete technology review and learn more about Aramus bag assemblies for high-density cell banking.
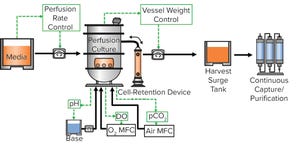
Figure 1: Vero cell expansion process
Anchorage-dependent cells such as Vero cells are used widely as a platform for viral vaccine production. Perfusion bioprocesses enable a constant addition of nutrients and removal of byproducts while cells are in a bioreactor. That results in cell densities that are higher than those for conventional batch or fed-batch processes. In the study herein, Eppendorf researchers tested the suitability of a spin filter as a cell-retention device. They cultivated Vero cells on Cytodex 3 microcarriers (10 g/L) in an Eppendorf 3-L glass vessel using a microcarrier spin filter coupled with a pitched-blade impeller. The microcarrier spin filter is a cylinder-shaped cage that spins with the impeller shaft and is covered with a large 75-μm screen designed to prevent microcarriers from being collected with waste media during perfusion. The process was controlled with a BioFlo 320 bioprocess control station. No additional devices were needed for the perfusion. With the unique design o...
The COVID-19 crisis has reinforced the importance of having a strong supply chain and a risk-management and business-continuity plan. How can you mitigate your raw material risk, especially during a pandemic?
Risks When Choosing a Raw Material Supplier:
How do you select your critical raw-material suppliers? What selection criteria and which risks have you identified? To understand risks related to supply, demand, material supplier capacities, and so on, you need information that can come only from communicating with your supplier about the stability of supply, production capacity, and transportation and distribution chain.
Fill out the form below to read the complete capabilities review and learn more about raw material risk mitigation now.
Figure 1: Typical configuration of WuXiUP platform
Recent world events have demonstrated now more than ever the growing demand for pharmaceutical biologics that can be made rapidly and in high volumes yet somehow remain affordable. Hence, there is an urgent need to develop a next-generation biomanufacturing solution that provides high-yield, high-quality drug products and is highly flexible and cost-effective. Herein we describe the WuXi Biologics ultrahigh
productivity platform (WuXiUP), an intensified perfusion culture process developed to meet the aforementioned need. WuXiUP adopts process-intensification strategies on to traditional perfusion culture processes to boost cell density and cell-specific productivity. The continuous harvest reduces greatly the residence time for a product within a bioreactor, leading to more desirable product quality and facilitating integrated continuous bioprocessing.
Fill out the form below to read the complete technology review now.
Sponsored Content
Never has there been a more critical time to be a company delivering technologies and expertise that helps get new biologics and vaccines to patients faster. Sartorius is that company. Working in partnership with scientists, we help turn research discoveries into real-world medicines. Our bioprocessing equipment and know-how are called on every day to create biologics that treat debilitating diseases, including cancer and arthritis, and make affordable vaccines to prevent common infections. When global organizations such as the Jenner Institute and CanSino Biologics need assistance accelerating vaccine production for pandemic viruses (e.g., Ebola, H1N1, and SARS-CoV-2), they turn to us.
Fill out the form below to read the complete capabilities review and learn more about single-use technologies for vaccine production.
Astrea Bioseparations Ltd. (Astrea) is the only bioseparations company to offer ligand discovery and adsorbent development services coupled with manufacture and supply of bulk quantities of chromatography adsorbents and production of prepacked chromatography columns to good manufacturing practice (GMP) standards. Our synthetic affinity ligands have been proven over 30 years through numerous examples of successful discovery, development, and delivery of novel affinity adsorbents. Our technology is designed to capture and purify selective target biomolecules — including recombinant proteins, human plasma proteins, fusion proteins, and engineered fragments — as well as remove specific impurities such as endotoxins, isoagglutinins, and prions.
Fill out the form below to read the complete capabilities review and learn more about synthetic affinity ligands now.
With 25 years’ experience in the biopharmaceutical industry, CPC proudly engineers single-use connection technologies that address biopharmaceutical manufacturers’ requirements for reliability, assurance of sterility, and ease of use.
CPC’s innovative line of genderless AseptiQuik sterile connectors allows manufacturers to make quick and easy sterile connections, even in nonclassified environments. All genderless AseptiQuik connectors feature an easy-to-use three-step process that greatly minimizes the risk of operator error and ensures that sterility will be maintained both before and after connection.
Fill out the form below to read the complete technology review now.
Figure 1: Endotoxin reduction efficiency of PURAFIX ET-R1 and ET-R2; filter sheets (21 cm
2
) were challenged with a PBS solution of 3 L, spiked with 5000 EU/mL.
PURAFIX ET-R depth-filter sheets are used for efficient and economical reduction of endotoxins from pharmaceutical liquids. Removal of endotoxins is one of the most important and challenging steps in biopharmaceutical processes. Endotoxins — more
precisely, lipopolysaccharides (LPS) — are extremely heat and pH stable and therefore withstand sterilization. Purification usually is performed by applying chromatography as a polishing step, which is costly and time consuming. By reducing endotoxin load at an early stage of a clarification process, subsequent purification steps are less challenging, and higher throughputs can be achieved, resulting in cost and time savings.
Fill out the form below to read the complete technology review now.
Figure 1: System and data communication diagram; historical data available in the data historian are used as reference batches and to build the digital chromatogram project. During production, data ingested from the manufacturing SCADA system to the data historian (OSI PI) are transmitted to SIMCA Online through SimApi at real-time frequency. The visualization dashboards are made available to operators, quality assurance personnel reviewers, and the partner.
Chromatogram review is a monitoring method used to verify process performance in packed-bed chromatography processes. By observing key process parameters such as chromatography column outlet conductivity or UV absorbance, it is possible to identify the signs of a poorly packed column, resin degradation, or equipment malfunction. Therefore, chromatogram review is implemented as an in-process control (IPC) to decrease variability and identify suboptimal performance, thereby enhancing yield and ensuring high product quality.
Fill out the form below to re...
Biopharmaceutical companies are facing major challenges today, including rising drug manufacturing costs and lower production volumes related to unpredictable demands. To achieve the necessary reductions in both costs of goods (CoG) and infrastructure and operational costs, biopharmaceutical manufacturers integrate process intensification strategies into their production schemes.
Novasep has embedded specific features into its BioSC™ platform to enable and accelerate process intensification (two key examples being viral inactivation steps
management and in-line buffer preparation).
BioSC Predict, Exclusive Simulation and Optimization Software:
BioSC Predict software facilitates and guides the switch from batch chromatography to optimized multicolumn chromatography. A deepened process understanding is becoming increasingly important to design a chromatographic separation method that consistently delivers high-quality products.
Fill out the form below to read the complete technology review now.
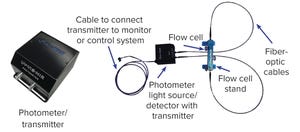
Figure 1: Turbidity system setup
Turbidity is the relative clarity of a liquid as the result of suspended solids. Typically, turbidity measurements are taken by using a beam of light to detect the presence of particles and measuring the difference between the amount of light that is emitted from a source and the amount received by a detector. In bioprocess operations, liquid turbidity often is measured postfiltration to detect “break through,” or undesired materials coming from a filter, which provides an overall assessment of filter performance.
Presently, turbidity measurements are made through offline sampling. However, that approach is inefficient and often disrupts bioprocesses. By contrast, PendoTECH’s
single-use turbidity measurement system takes on-line measurements, enabling users to monitor turbidity in real time. The system uses single-use flow cells, which enable users to take noninvasive measurements (no physical product contact), and it eliminates the need for typical maintenance requirement...
Frozen storage of bulk drug substance (BDS) at temperatures at or below –70°C is common in the bioprocess industry. Storage at temperatures below –80°C can lead to spontaneous failure of typical BDS containers, particularly if container temperature is reduced rapidly (known as flash freezing).
Most containers used to store BDS have glass-transition temperatures well above –196°C, and many containers structurally fail during rapid descent through glass transition. Even worse, such failures often are detected after a container is thawed, which can take weeks to months after freezing is completed.
By contrast, materials composed of fluoropolymers typically do not change structurally when flash frozen. Thus, a container system manufactured from fluoropolymers can not only survive flash freezing, but also retain the same functionality it had when at room temperature.
Fill out the form below to read the complete capabilities review now.
Figure 1: SONOFLOW CO.55 flow meter for noncontact flow monitoring (LEFT) and SONOCHECK ABD06 sensor for bubble detection (RIGHT)
Noninvasive SONOFLOW clamp-on flow sensors and noninvasive SONOCHECK bubble detectors are designed for accurate and contamination-free upstream and downstream monitoring to fulfill regulatory goals within the process analytical technology (PAT) framework.
Field-Proven Technology:
SONOFLOW clamp-on flow sensors are designed for upstream and downstream bioprocess monitoring. These innovative sensors have
integrated electronics that enable them to function without an external board or transmitter, providing a complete flow meter in a device the size of a small transducer. The systems can be applied throughout process development, manufacturing, and fill–finish operations.
Fill out the form below to read the complete technology review now.
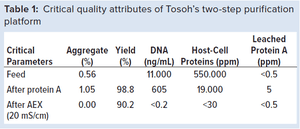
In this study, we showcase the benefits of Tosoh’s two-step process for the purification of monoclonal antibodies (MAbs) in comparison with the standard industrial process. Combining high-performance protein A capturing and a single polishing step on a salt-tolerant anion-exchange resin, we could reduce the downstream costs by 45% and increase production output by 58%.
TOYOPEARL AF-rProtein A HC-650F is a high-capacity protein A resin for the purification of MAbs. This resin exhibits dynamic binding capacities (DBC) of 70 g/L at five minutes of residence time.
Fill out the form below to read the complete technology review and learn more about TOYOPEARL resins now.
Sponsored Content
AbbVie is a global, research-driven biopharmaceutical company committed to developing innovative advanced therapies for some of the world’s most complex and critical conditions. The company’s mission is to use its expertise, dedicated people, and unique approach to innovation to markedly improve treatments across four primary therapeutic areas: immunology, oncology, virology, and neuroscience. In more than 75 countries, AbbVie employees are working every day to advance health solutions for people around the world. Combining the focus of a biotechnology company with the expertise of an established pharmaceutical leader, a contract manufacturing organization (CMO) is agile and flexible in its service to companies outsourcing the development and manufacture of drugs.
Fill out the form below to read the complete capabilities review now
.
Figure 1: Capabilities flow chart
As a result of increased focus on customized medicine and continued emphasis on drug
development for rare diseases, the biopharmaceutical industry continues to advance its methods for disease treatment by developing target-specific novel therapeutics.
Formulations for these types of programs are complex and therefore present challenges in the design, scale-up, and manufacture of drug substances and drug products. Such challenges can delay getting critical products to patients. Contract development and manufacturing organizations (CDMOs) can help advance therapeutics and navigate production hurdles that traditionally can slow those programs down.
Ajinomoto Bio-Pharma Services is your trusted CDMO partner, providing a broad range of capabilities, regulatory excellence, and extensive experience to help you navigate those challenges, provide solutions to your development process, and deliver your new therapies to patients who need them.
Fill out the form below to read the com...
byJB Agnus
Avid Bioservices is a full-service, dedicated contract development and manufacturing organization (CDMO) focused on development and manufacturing of biopharmaceutical drug substances derived from mammalian cell culture. Avid’s biologics development and manufacturing services include process development and current good manufacturing practice (CGMP) clinical and commercial drug substance manufacturing, bulk packaging, release and stability testing, and regulatory submissions support. For early stage programs, the company provides various process development activities, including upstream and downstream development and optimization, analytical methods development, testing, and characterization. Avid has 27 years of experience producing a comprehensive range of proteins, including monoclonal antibodies (MAbs) and recombinant proteins as well as bispecific monoclonal antibodies, Fc-fusion proteins, and biosimilars. The scope of our services ranges from stand-alone process development projects to full developm...
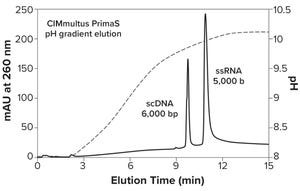
Figure 1: High-resolution separation of pDNA from ssRNA; double-stranded dsRNA also elutes in advance of ssRNA.
CIMmultus PrimaS multimode ligand bioprocessing technology represents a new class of anion exchangers for fast, efficient, one-step purification of single-stranded mRNA at ambient temperature. DNA, dsRNA, and proteins are eliminated with a high-salt wash. Then ssRNA is fractionated by size in a pH gradient that removes short transcripts and fragments. Convective mass transfer and laminar flow through the technology’s monolithic architecture maintain high capacity, high resolution, and low shear stress — even at flow rates >5 column volumes per minute.
Fill out the form below to read the complete technology review and learn more about CIMmultus PrimaS technology now
.
Gene therapy holds the promise of treating the unmet needs of patients who suffer from rare genetic diseases. An estimated 4,000 medical conditions are a result of gene disorders with no established targeted treatments. Gene therapy offers new options away from the conventional symptomatic approach to disease treatment as well as provides hope for real cures.
At Catalent Cell & Gene Therapy, we understand the unique and complex nature of bringing a gene therapy to market, and we are dedicated to achieving the best approach for your product from start to finish. As unique as the therapies themselves are, every path from the bench to the clinic is tailored to ensure that your results are timely and efficient. We are here to optimize your viral development and streamline your manufacturing process for optimal and reliable titers to achieve a stable and effective product dose.
Fill out the form below to read the complete capabilities review about Catalent Cell & Gene Therapy now.
Emergent BioSolutions is a global life sciences company whose mission is to protect and enhance life. Through our specialty products and contract development and manufacturing services, we are dedicated to providing solutions that address public health threats. Through social responsibility, we aim to build healthier and safer communities. We aspire to deliver peace of mind to our patients and customers so that they can focus on what’s most important in their lives. By working together, we envision protecting or enhancing 1 billion lives by 2030.
This capabilities review explores the CDMO services of Emergent Biosolutions. Fill out the form below to read the complete article now.
Rentschler Biopharma is an international contract development and manufacturing organization (CDMO) dedicated to biopharmaceutical development and biomanufacturing for over 40 years. We are leading experts with an exceptional track record of over 100 different therapeutic protein formats. As a world-class solution provider, we ensure optimum time to proof-of-concept for clinical stages I and 2, as well as time to market for products in clinical stage 3. By accelerating timelines under consideration of economically attractive process options, we generate a competitive advantage for our clients, with no compromises regarding product quality and safety.
Fill out the form below to read the complete capabilities review now.
Richter-Helm is a Hamburg, Germany–based contract manufacturing company with a proven 30-year track record and specialized in products derived from bacteria and yeasts. Count on us to flexibly provide a comprehensive range of services and customized solutions. Clients worldwide already have benefited from our commitment to good manufacturing practice (GMP) and total transparency. Our work focuses on recombinant proteins, plasmid DNA, antibody fragments, and vaccines.
Our seasoned, 240-strong team supports you with process development, supply of products for clinical trials, commercial production, in-house quality control (QC) testing, and QP release. We operate two GMP-compliant production plants with bioreactor capacities of up to 1,500 L.
Richter-Helm consistently works to the highest standards of pharmaceutical quality, as verified by major regulatory bodies, including the European Medicines Agency (EMA), the US Food and Drug Administration (US FDA), Agência Nacional de Vigilância Sanitária (ANVISA), a...
Figure 1: Leachables risk evaluation
Single-use technologies (SUTs) can introduce leachables (chemicals that migrate from polymeric components into the drug product) to a manufacturing process. Herein, we describe an effective process to manage the risk from leachables to ensure patient safety and prevent delayed product launch. Early implementation of leachables risk evaluation strategies can reduce the resources and time required for risk mitigation later in a project’s life cycle.
Fill out the form below to read this technology review now.
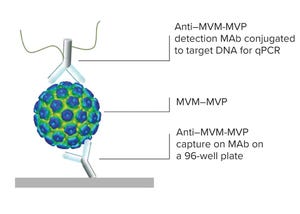
Figure 1: Immuno qPCR
Viral contamination is an inherent risk during the manufacture of therapeutic products such as antibodies, vaccines, viral vectors, and plasma derivatives. Whether introduced endogenously from raw materials or exogenously through manufacturing operations, unmitigated viral contaminations can lead to serious health implications and facility shutdowns. Thus, international regulatory agencies require sponsoring companies to validate the “viral clearance efficacy” of their downstream purification process steps before clinical trials or commercial approval.
This technology review describes the MockV MVM kit for viral clearance prediction. Fill out the form below to read the complete article now.
Octet platform poster
Using the label-free optical technique of Bio-Layer Interferometry (BLI), the Octet platform provides real-time analysis of molecular interactions. It relies on the robust and easy-to-use Dip and Read™ format, which provides faster time to results relative to technologies like ELISA and SPR. It also operates in a fluidics-free format, thereby minimizing the complexity in analyte detection by fluidics-based technologies like SPR. It provides high-throughput analysis, with the option of analyzing to 96 samples simultaneously, thereby increasing analytical productivity. It has high tolerance to a diverse array of sample types, making it compatible with in-process testing during drug substance manufacturing. In addition, the samples on analyzed on an Octet system can be reused for other analysis, minimizing depletion of precious samples and maximizing process economy. In this poster, we will describe multiple ways in which the Octet platform is well suited for GxP and QC applications.
Fi...
Figure 1: Structural elements of messenger RNA
Messenger RNA (mRNA) therapeutics have the potential to revolutionize several areas of medicine, including the prophylaxis of infectious disease. That potential is driven not only by therapeutic advantages of this modality, but also the relative ease in which a product can be produced and scaled — thus reducing cost and importantly, time to market. mRNA therapeutics have an enhanced safety profile driven in part from their mode of action not requiring integration into the host-cell genome.
mRNA vaccines are a relatively new concept, and little regulatory guidance is available on product characterization or quality control. To support mRNA vaccines through development and to market — ensuring safe and efficacious vaccines — thus requires a strategic science-led program to be implemented that identifies critical attributes and enables their evaluation and, ultimately, their control.
Fill out the form below to read the complete technology review now.
Bio4C™ ProcessPad data visualization, analytics, and process monitoring platform enables bioprocess life-cycle management, reporting, investigations, and continued process verification (CPV). The system intelligently combines process data from disparate sources into a single, validated data source. The Bio4C ProcessPad platform also facilitates 21 CFR Part 11 compliance and ensures access within a validated environment to verified process data that is current and contextual throughout a product life cycle.
Fill out the form below to read the complete technology review now.
Countless factors influence a bioprocess, and measuring and evaluating them can be a major challenge. One of the most important performance parameters is the volumetric mass-transfer coefficient (
k
L
a
). ZETA has developed a reliable method for determining the
k
L
a
value and thus has revolutionized bioreactor design in plant engineering. With ZETA’s mass-transfer coefficient value determination system, measurements to determine
k
L
a
value can be taken at any point in a bioreactor, and based on such measurements, production conditions can be mapped perfectly.
Fill out the form below to read the complete technology review now.