September 2020
Brian Caine, Publisher,
BioProcess International
from 2002-2020
By the time you read this, I will have officially called it a career at
BioProcess International
. Over 18 years — where did the time go? In many ways it feels wrong to be writing this — too soon, with too much still to do. But deep down, I know the timing is right. Professionally, it’s time to turn the reins over to the competent, experienced, and tireless staff you already know — either personally or through the pages of
BioProcess International
— and provide them with the opportunity to take your favorite industry publication to the next level. Personally, I have been in the life-science publishing business for over 24 years. And although I have enjoyed every minute on the job, it’s time to try something new.
But what a ride! How often can you be a part of starting a business from scratch; pouring in blood, sweat, and tears; not knowing if you will survive the legal challenges (a story for another time), if the market will accept your...
WWW.COPPERHOUNDPICTURES.COM
I’m writing this from the height of our pandemic summer, and you’re reading it in the fall — in this strange year of time flying while seeming to stand still. We editors have worked from home for years, so our lives haven’t changed as much over the past few months as yours might have. Our Informa conference organizers, however, have seen their jobs transform radically. They’ve managed to pivot deftly toward virtual events, and I hope you’ve enjoyed some of those by now. We sure do miss seeing our readers and authors in person, but the lack of physical travel can allow us all to tune in to more discussions than we might have otherwise.
We’ve been thinking of you a lot lately, too. Late summer is when the BPI editors, publisher, and sales staff begin planning our schedule for the next year. Of course, we consider what topics are getting a lot of your attention this year — hmmm, vaccines, anyone? — but we also like to get ahead of the trends when we can. That can be a challenge. S...
Figure 1: Basic principles of technology transfer
In the biopharmaceutical industry,
technology transfer
refers to transfer of any process, together with its documentation and professional expertise, between development and manufacture or between manufacturing sites (
1
). This operation is common in the biopharmaceutical industry for a number of structural reasons. They include the dichotomy between small, innovation-based drug companies and large ones able to conduct late-phase clinical development and endowed with manufacturing capacity; the high capital cost of biopharmaceutical plants, which makes contract manufacturing attractive; and the need for multiple scale-ups during a product’s life cycle.
“Tech transfer” is a delicate operation carrying business, regulatory, product quality, and technical risks. The basic principle is simple enough: Originators transfer all their know-how to recipients to enable product manufacture. Even in that basic rendering, some difficulties are detectable: It is not ...
The CASSS chemistry, manufacturing, and controls (CMC) Strategy Forum on 23 January 2019 in Washington, DC, was entitled, “The Development of Patient-Focused Commercial Specifications Through Understanding of Clinical Relevance and Criticality of Quality Attributes.” This forum covered the definition, identification, control, and management of patient-focused attributes throughout the life cycle (from discovery through approval) of biological products, including vaccines. Participants investigated how to differentiate through the product development life cycle which attributes are “clinically meaningful” from those applied for manufacturing capability and/or process consistency. Speakers explored which attributes have been identified as critical quality attributes (CQAs) and which have been shown to have no clinical relevance in addition to how those determinations were reached.
In addition, participants discussed developing a life-cycle approach to clinically meaningful specification development. Approac...
Tremendous growth in the cell and gene therapy (CGT) industry is driving unprecedented demand for manufacturing services. To be sure, advanced-therapy developers increasingly are choosing to install in-house capabilities. Doing so can offer companies greater control of their processes, timelines, and budgets than they might have when outsourcing products (
1
). But industry experts agree that contract development and manufacturing organizations (CDMOs) will remain integral to CGT manufacturing and commercialization (
1
,
2
), especially with veteran contract partners scrambling to acquire CGT capability and expertise.
As part of Cell and Gene Therapy Bioprocessing and Commercialization Virtual (19–22 October 2020), Vadim Klyushnichenko (vice president of pharmaceutical development at
Calibr
) will explore how CGT developers should select contract partners. Klyushnichenko oversees contract partner management, process development, good manufacturing practice (GMP) manufacturing, quality assurance, and sup...
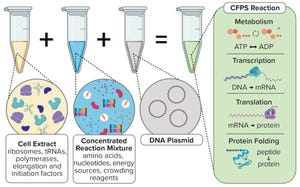
Components of a cell-free protein synthesis reaction (extract, supplements, and a DNA template) with the key reactions that occur when they are combined
Cell-free synthesis (CFS), also known as
cell-free transcription and translation
, supplements cellular components (either a cell lysate or purified recombinant elements) with nucleotides, amino acids, metabolic intermediates, and salts to produce a nucleic acid or protein from a genetic template added to the reaction. This exciting technology has seen a substantial increase in both academic and commercial interest over the past decade (
1
). Interest stems in large part from the potential to democratize access to the machinery of biology by removing the need to engineer cells genetically (
2
). CFS has the potential to revolutionize healthcare in much the same way that personal computers did for information technology (
3
). However, our interest and that of the bioprocessing community at large comes from the potential to transform manufacturing for som...
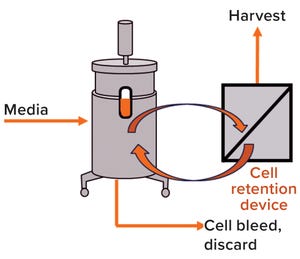
Figure 1: Simple schematic overview of a mammalian cell culture perfusion system
Perfusion cell culture processes are continuous, with fresh media continuously added and spent media (harvest) removed simultaneously through a cell-retention device (Figure 1). To maintain specific bioreactor cell density, cells are removed periodically as cell bleed or discard.
Perfusion systems offer a number of advantages over batch and fed-batch culture modes such as lower capital costs and an ability to support higher cell densities with better viability over longer manufacturing campaigns requiring shorter turn-around times. However, perfusion systems require complex operations monitoring and control because of their cell-retention systems.
At a steady state, a cell culture perfusion system has attained a target cell density with constant parameters such as media and harvest flow rate, cell viability, and pH and O
2
/CO
2
levels. At this point, typically maintained throughout the production campaign, cells are expecte...
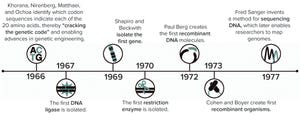
Figure 1: Early advances in DNA understanding were foundational for protein overexpression.
Since the dawn of the recombinant DNA era in the 1970s, New England Biolabs (NEB) has been integrally involved in expressing and purifying proteins, both for its own research interests and for biomanufacturing processes. In 1978, the company began screening microorganisms for restriction enzymes. Our scientists remember the challenges met in purifying limited amounts of restriction enzymes and other proteins from native organisms isolated from the environment. The efforts of those scientists to clone, overexpress, and purify restriction enzymes from recombinant systems helped to advance the field of molecular biology. Many of their original methods have endured to be applied by other scientists in studying the structure and function of individual proteins. Now, NEB scientists are striving to develop rapid, simplified methods for recombinant protein expression and purification that rely on engineered protein express...
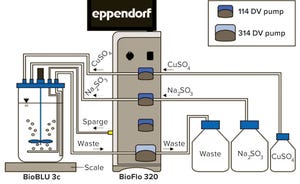
Figure 1: BioFlo 320 experimental setup
Proper tuning of dissolved oxygen (DO) controller proportional integral (PI) values is essential for optimal cell culture performance in a bioreactor. When DO-PI values are optimized, gas flows are smoothed, and foaming and cell stress are reduced. Traditionally, this tuning has been performed by using nitrogen gas to purge oxygen from a test solution, thus simulating oxygen demand. That method has several drawbacks, however. First, nitrogen gassing cannot simulate the high demands of high-density fermentation. Second, nitrogen competes with other gases, especially in single thermal mass-flow controller (TMFC) systems. The artificial gassing interference is not normally present in a cell culture. And third, nitrogen gassing cannot be used to test four-gas control mechanisms in which nitrogen is used to balance DO as part of the gassing strategy.
Equation 1: Reaction formula
Here, we describe a method of simulating DO demand using chemical oxygen scavengers such as s...
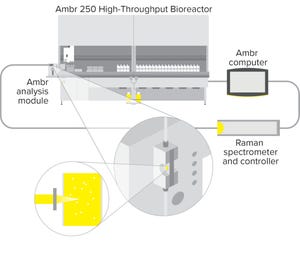
Figure 1: Schematic diagram of the Ambr analysis module showing the BioPAT Spectro platform fitted to an Ambr 250 high-throughput system
Raman spectroscopy is used widely in biomanufacturing as a process analytical technology (PAT) for monitoring analytes such as glucose and lactate (
1
). Predictive Raman models also can be used to control glucose concentration in cell cultures (
2
). The technique is becoming more popular for pilot- and manufacturing-scale bioreactors, but it only recently has been studied with minibioreactors for measuring analytes and producing predictive Raman models for feedback control (
3
) thanks to advances in integrated technology for automating sampling, analysis, and data management.
Raman-based process control relies on developing calibration models that correlate a spectral signal with an analyte or other measurements, generally from benchtop- to production-scale bioreactors. To build such a model requires measuring multiple analytes, ideally in a design of experiments (DoE...
Gastric delivery is unachievable for most biopharmaceuticals, so drug developers formulate biologics primarily for intravenous infusion or injection. However, inhaled and nasal delivery options are attracting considerable attention because they enable targeted delivery of a wide range of therapeutic proteins. On 23 June 2020,
Mark Parry
(technical director at Intertek) presented an “Ask the Expert” webinar that described critical considerations for inhaled and nasal delivery for biologics.
Parry’s Presentation
Because biopharmaceuticals are complex products, developers need compelling reasons to choose inhaled and nasal formulations. Targeted drug delivery is one such reason. As researchers have observed with therapeutics for asthma/chronic obstructive pulmonary disease (COPD) and antibiotics, local administration facilitates delivery of therapeutic doses. That enables developers to reduce the amount of active pharmaceutical ingredient (API) in each unit, often improving a drug’s side-effect profile.
Re...
In his 28 May 2020 “Ask the Expert” presentation,
Jeroen Geeraerts
(business development manager at Rousselot) highlighted that, as of May 2020, 118 candidate vaccines for the novel coronavirus (SARS-CoV-2) had reached clinical or preclinical evaluation. Of those, 11 use inactivated and live–attenuated approaches, which require strong stabilizing agents to maintain vaccine potency. Geeraerts explained why inactivated and live–attenuated vaccines require stabilizing agents and why vaccine companies prefer pharmaceutical-grade gelatin for such applications. Next, Geeraerts described how his company’s X-Pure highly purified gelatin excipients can preserve vaccine potency in a low-endotoxin format.
Geeraerts’s Presentation
Geeraerts explained that subunit/recombinant and toxoid vaccines feature particles (microbial fragments and toxins, respectively) that remain stable without excipients. However, inactivated and live–attenuated vaccines need to deliver whole virus particles, which require support to remain...
Distinguishing aggregated active pharmaceutical ingredients (APIs) from other particle types is critical to evaluating a protein product’s stability, but standard characterization tools struggle to discriminate proteins from nonproteins with similar sizes, shapes, and morphologies. In a 25 June 2020 “Ask the Expert” webcast,
Bernardo Cordovez
(founder and chief scientific officer of Halo Labs) introduced his company’s Aura subvisible-particle analyzer. He explained how the device combines an innovative microscopy technique with sophisticated imaging software to characterize subvisible particles more quickly and accurately than incumbent technologies can.
Cordovez’s Presentation
Cordovez observed that flow imaging requires large sample volumes and cannot distinguish proteins from plastics or degraded polysorbates. Raman and Fourier-transform infrared (FTIR) spectroscopy can do so but require considerable time to acquire spectra and significant expertise to determine which signals to isolate and analyze. ...
The US National Cancer Institute (NCI) has launched the Proteomic Data Commons (PDC), a next-generation proteomic data repository that will facilitate data access, sharing, and analysis to speed development of precision-medicine therapies for cancer. Housed within the NCI’s Cancer Research Data Commons (CRDC), PDC hosts several multiomic data sets that have corresponding genomics and imaging data elsewhere, simplifying their access to enable integrative research.
Increase Access to Enable Discovery
Mass spectrometry (MS)–based proteomic profiling for pancancer analyses could enhance our understanding of cancers’ underlying genomic events and help identify potentially meaningful changes at the proteomic level that otherwise are missed at the genomic level. Currently, researchers’ ability to access such diverse data sets and perform robust and reproducible analyses is stifled by the siloed nature of the informatics infrastructure. Given that public money funds most cancer research, it is imperative to make ...
Chinese hamster ovary (CHO) cells have emerged as a robust platform for bioprocessing serving both early and late-stage biotherapeutic drug supply. However, these cells and other hosts (e.g., HEK293), can be optimized for even greater potential through advanced gene editing. For example, when the endogenous glutamine synthetase (GS) gene is knocked out in CHO cells, a sixfold increase in high-producing cell lines is achieved (
1
). In another study, CHO with annexin A2 (ANXA2) and cathepsin gene (CTSD) knockouts were introduced to eliminate CHO host-cell proteins that complicate downstream processing and can contribute to immunogenicity (
2
).
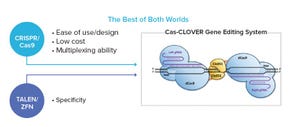
In this BPI supplier-side article, Demeetra AgBio introduces the Cas-CLOVER gene-editing technology, a dimeric nuclease system that, unlike CRISPR/Cas9, does not result in detectable off-target mutagenesis.
Fill out the form below to read the full article now.
References
1
Fan L, et al. Improving the Efficiency of CHO Cell Line Generation Using Glutamine Syntheta...