
A shortage of bioprocess talent drives high salaries and hinders drug development, says BioPlan Associate’s Eric Langer. As biologics increasingly dominate pipelines, biomanufacturers look to traditional pharma to plug the staffing gaps.
Speaking last month at the BIOLive event at CPhI Worldwide in Madrid, Eric Langer, president and managing partner at BioPlan Associates, said hiring is one of the biggest problems facing the biopharma industry.
“It is painful [to the biopharma hiring managers] that 40% of the biopharma industry cannot hire the process development people it needs. So what does that do to the supply and demand curves? If I was advising my kids now on where they want to be in their career I would say biopharma engineering is probably a real good spot because you command your salary at this point. They cannot find enough of you.”

Image: iStock/Darwin Brandis
And while some Big Biopharma firms will open their wallets to plug these gaps by poaching staff from other companies, he added “there are blockbuster biologics out there that are not being developed because they don’t have somebody at hand to be able to do that.”
Looking to small molecule makers
Staff poaching is one way to solve the issue, but it’s a short-term solution and the raising of salaries increases the overall manufacturing costs, which decreases the profitability of the biologic being produced. Therefore, Langer said biopharma is looking at its traditional pharma counterpart.
“We are going to see is a lot of expertise coming over in the hiring area, that’s going to be an area where we will see a lot of the convergence,”
His comments are supported by results from BioPlan Associate’s 15th Survey of Biopharmaceutical Manufacturing Capacity and Production, published earlier this year, which high-lights the high demand for bioprocessing expertise. The report shows biopharma firms have been increasing both their training and hiring budgets over the past few years, and at the same time firms are hiring more from small molecule pharma.
Nearly 23% of survey respondents hired scale-up or process development, and quality management from traditional pharma, while around 20% hired regulatory compliance, engineering facility design, general R&D and quality control testing staff from the small molecule pharma pool.
“What we wanted to see [in the survey] was where could – and where are – Biopharma hiring,” he said, adding what was found were a long list of perceived portable skill categories viewed by biomanufacturers.
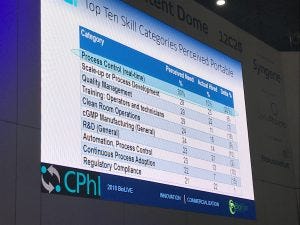
A slide taken from Eric Langer’s presentation at CPhI Worldwide last month
Training support
Langer’s views were mirrored by Killian O’Driscoll, director of projects at Ireland’s National Institute for Bioprocessing Research and Training (NIBRT).
“The demand for talent is quite acute in some areas, in particular the specialist engineering positions such as experienced bioprocess engineers, CQ+V engineers,” he told BioProcess Insider. “This demand is likely to intensify as more complex therapies are manufactured using a diverse array of advanced manufacturing technologies.”
Located in Dublin, NIBRT has been described as a ‘flight simulator for biomanufacturing’ and – since it opened in 2011 – trains over 4,000 people per year in bioproduction processes.
“A sizeable portion of these would have small molecule experience,” O’Driscoll said. “A significant but very achievable effort is required for an experienced small molecule employee to develop the additional competencies required for biopharma manufacturing. NIBRT has provided biopharma manufacturing ‘cross training’ to many clients from the small molecule sector who have subsequently gone onto successful careers within biopharma.”
One example he cited was Bristol-Myers Squibb (BMS) at its biologics site in Cruiserath, Dublin. The firm used NIBRT to cross train selected employees from its existing small molecule facility.
But generally companies will need to assess all potential sources of talent, he said. “Those who have small molecule manufacturing experience have many of the key competencies required to make a successful transition into biopharma manufacture as they have the skills and experience of working in GMP regulated environment. However, there is a requirement to cross skills on the key competencies need for biopharma such as aseptic manufacturing, cell culture, complex bioanalytics, protein purification etc.”
About the Author
You May Also Like

schedl_b_and_w.jpg?width=100&auto=webp&quality=80&disable=upscale)
schedl_b_and_w.jpg?width=400&auto=webp&quality=80&disable=upscale)


