Performance Qualification of a Single-Use, Stirred-Tank Bioreactor with CHO-S Cell Culture

Photo 1: Pall Allegro STR 200 bioreactor
The increasing role and importance of cell culture in biophamaceutical manufacturing has led to considerable research and development (R&D) into bioreactor design and performance in recent years. As a result, a greater understanding of bioreactor fluid dynamics and critical physical parameters is now essential to maximize cell growth and productivity. Stirred-tank bioreactors are especially important in this development process because of their favorable properties in areas such as mixing efficiency and homogeneity, energy transfer, and scalability.
The design and manufacture of single-use bioreactors is another major area of R&D. The objectives have been to achieve and (when possible) improve fluid dynamics and cell culture performance of stainless steel systems but in a single-use, presterilized format. This alternative technology also offers product developers an opportunity to create and manufacture innovative designs not achievable with multiuse cleanable systems. That can provide unique benefits to users and offer process enhancements (1).
In a previous publication (2), we described the design features of a single-use, 200-L, stirred-tank bioreactor (Photo 1) based on a customized, cubical biocontainer with internal baffles and a large, direct drive, pitched impeller with three blades. Data from our studies on the bioreactor’s fluid dynamics showed that the system could provide
Good power transfer from the direct drive impeller for volumes ≤200 L
Rapid and homogeneous mixing
Mixing predictions from fluid dynamics computer modeling similar to data obtained experimentally
High oxygen mass transfer (kLa O2) and CO2 stripping rates using ring and open-pipe spargers
Precise and homogenous control of temperature.
Having established that the fluid dynamics satisfied critical requirements for mammalian cell culture, we carried out further studies to evaluate its cell culture performance using a Chinese hamster ovary (CHO)-S cell line and its comparative performance and scalability for cell culture studies against traditional stirred-tank bioreactors. Herein we present study results and the signficance of those results showing
Good cell density and viability, monoclonal antibody (MAb) productivity, pH control, glucose consumption, and nutrient profiles
Cell culture performance equal to or better than a conventional 10-L glass multiuse bioreactor
Full scalability to 200 L.
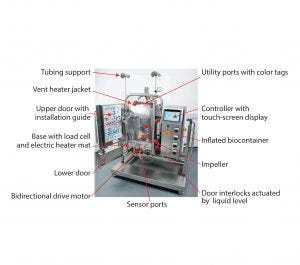
Figure 1: Pall Allegro STR 200 bioreactor components
Single-Use Bioreactor Design and Functions
These studies used a Pall Allegro STR 200 bioreactor. Full details of the single-use components and associated hardware are described in a previous publication (2) and in detailed product information published by Pall (3). So we briefly summarize the design features here. Figure 1 shows the complete assembled system, and the box summarizes its main components and functions.
Components and Functions
The Pall Allegro STR 200 single-use bioreactor has the following components and functions. |
Biocontainer: cubical format, lowdensity polyethylene, high-strength welded seams, low extractables, singlepiece system, gamma sterilized, automated in-place inflation, 60–200 L working capacity, integrity tested in factory, internal baffles, large threebladed impeller, bottom-mounted direct drive, reversible upflow and downflow |
Air and Gas Portal: aeration ports, ring sparger, gas port for overlay, two sterilizing gas filters, open pipe sparger, two vent ports, two sterilizing vent filters, vent filter heater jackets |
Liquid and Sensor Portal: Nine liquid input ports, two sampling ports, drain ports, temperature sensor port, four other sensor ports, sterilizable insertion bellows, color coding for utilities, numbered installation tags |
Hardware: stainless steel support, automated filling and dosing, load cell for filling and dosing, automated heater pad, liquid-level door lock |
Controls and Data Transfer: customized controller, touchscreen interface, password/user rights control, data in protected format, 21 CFR Part 11 compatible, OPC server for data transfer, linkable to SCADA systems, optional manual USB transfer |
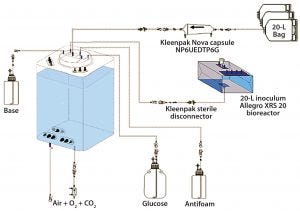
Figure 2: Experimental set-up for comparative cell culture study.
Studies on Cell Culture Performance
After we established that the engineering and fluid dynamics of the Allegro STR 200 single-use bioreactor met the requirements for mammalian cell culture (2), we carried out further studies to evaluate cell culture performance. Those studies were designed not only to measure cell growth rate and viability, but also to generate comparative data from a conventional, cleanable, stirred-tank bioreactor. That way, we could establish whether a single-use system could offer comparable performance and scalability to a multiuse system.
Experimental Set-Up: Figure 2 shows the set-up for our study. Full experimental details are available in a separate publication (4), so we summarize only the main components and practical details as follows:
CHO-S cell line producing a human IgG antibody
Chemically defined medium with added glucose and antifoam
10-L Eppendorf CelliGen 310 glass bioreactor (×2)
200-L Pall Allegro STR 200 single-use bioreactor
20-L Pall Allegro XRS 20 single-use bioreactor for cell harvest and inoculum
Oxygen, pH, and CO2 sensors and probe bellows
BioProfile FLEX analyzer
Sterile connectors and disconnectors
Sterilizing liquid- and gas-filter capsules
Sterilizing vent filters with heater jackets.
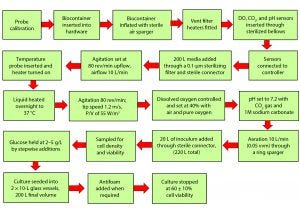
Figure 3: Procedures for cell culture in Pall Allegro STR 200 bioreactor
Our experimental approach was designed to ensure that culture conditions were the same for both the 10-L glass bioreactor and the 200-L single-use bioreactor and that critical physical and biological parameters could be monitored to provide a meaningful performance and comparative study. Figure 3 shows the sequence of unit operations for cell culture in the Pall Allegro single-use bioreactor.

Table 1: Control set points for comparative studies on bioreactors
For the comparative studies, we transferred 20 L of culture fluid from the Allegro STR 200 bioreactor after a 24-hour culture into the two 10-L Eppendorf CelliGen 310 glass bioreactors. We set the agitation for the glass bioreactors at a rate giving a similar tip speed to the Allegro STR 200 bioreactor of 1.2 m/s. Table 1 lists the control set points for both bioreactor types. We sampled the bioreactors over the culture period for cell density, culture viability, MAb titer, pH, and levels of lactate and glucose.
Results and Discussion
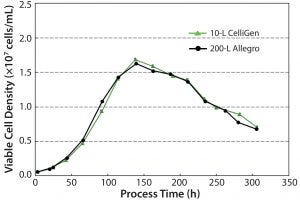
Figure 4: Viable cell density over time
Cell Growth: Figure 4 shows viable cell density profiles for the 10-L and 200-L bioreactors. The following conclusions could be drawn from the data:
Cell growth was similar at the 10-L and 200-L scale.
After a short lag phase of ∼24 hours, cells proliferated exponentially.
Cell densities peaked at 16.3 × 106 and 16.7 × 106 at the 10-L and 200-L scale, respectively.
Decline phase was at a similar time point of 140 hours for both bioreactors.
Similar cell death rates were exhibited up to the end of culture.
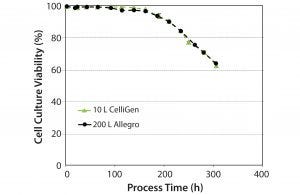
Figure 5: Cell culture viability over time
Culture Viability: Figure 5 shows culture viability profiles for the 10-L and 200-L bioreactors. We obtained similar profiles at both the 10-L and 200-L scales. For the Allegro STR 200 bioreactor with an agitation speed of 80 rev/min, a P/V power value of 55 W/m3 (5), and calculated tip speed of 1.2 m/s, results showed high cell densities and viabilities, indicating no evidence of cell damage.
With results of the cell growth studies, we concluded that the single-use Allegro STR 200 bioreactor produces culture profiles comparable with those of a conventional multiuse bioreactor, and it is scalable up to 200 L.
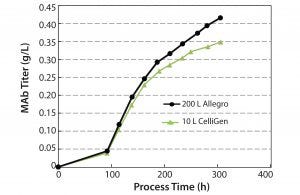
Figure 6: Monoclonal antibody titer profile
MAb Production: In addition to conducting studies on cell growth profiles, we investigated bioreactor productivity by measuring MAb titers (Figure 6). The mean titer at the end of the culture period for the CelliGen bioreactors was 0.35 g/L compared with a slightly higher titer of 0.42 g/L for the Allegro bioreactor. Results for productivity established that the single-use system could achieve equal or better MAb titers for batch sizes ≤200 L.
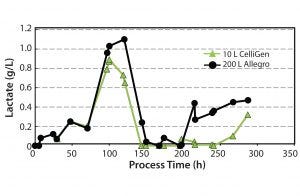
Figure 7: Lactate concentration profile
Cellular Metabolism and Control of pH: We investigated cellular metabolism by monitoring levels of lactate and glucose. Lactate accumulation can lead to acidification of the media, whereas consumption of lactate usually correlates with an increase in pH. By measuring pH, we can establish whether the bioreactors were able to effectively manage control of pH, which is vital in maintaining cell growth and viability.
Figure 7 shows lactate levels measured. Lactate levels showed significant variations throughout the culture period for both types of bioreactor. Despite those variations, pH control (set with a deadband of 0.05 units) efficiently maintained the set point of 7.2 ± 0.1 at both the 10-L and 200-L scale.
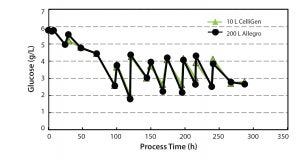
Figure 8: Glucose concentration profile
Control of Glucose Levels: Glucose was the primary nutrient for the CHO cell cultures. Effective control of glucose levels was a critical parameter to evaluate in our studies. We set glucose
concentration level at 2–5 g/L and controlled it by regular step additions throughout the culture period. Figure 8 shows that glucose concentrations were controlled to the required levels at both the 10-L and 200-L scale.
Performance Summary
In earlier studies (2), we measured a range of critical parameters and established that a single-use stirred-tank bioreactor could provide necessary fluid dynamics for mammalian cell culture, including
Homogeneous mixing in <10 seconds
Sufficient power input for fluid volumes ≤200 L
Good oxygen mass transfer (kLa O2) through the ring sparger
Effective CO2 stripping (6) using an open pipe sparger
Precise and homogeneous temperature control across the bioreactor volume.
Studies reported herein were designed to establish whether such physical attributes could provide equal or better cell culture performance to a conventional multiuse bioreactor as well as scalability up to a 200-L capacity. CHO-S cell culture studies showed that for the Allegro STR 200 single-use bioreactor, cell density and viability, MAb productivity, pH control, glucose consumption, and lactate profiles were all within the expected performance parameters for the CHO-S cell line. This performance was equal to or better than that obtained with the CHO-S cells in a conventional 10-L glass multiuse bioreactor. Data also confirmed full scalability to 200 L for the single-use bioreactor.
One of the most challenging requirements when designing single-use bioreactors has been the need for high-power transfer capacity. Our studies have shown that the direct-drive, elephant-ear impeller of the Allegro STR 200 bioreactor could generate high P/V power values of 310 W/m3 (2, 5). That feature can provide considerable flexibility in control of cell culture conditions and could be particularly valuable in applications requiring high cell densities or viscosities.
We hope this performance qualification data for the Pall Allegro STR 200 bioreactor will be helpful in operations where the introduction of single-use bioreactor technology into new processes is being considered or where replacement of existing stainless steel systems is under review. The data may also help reduce lead times for implementing a single-use system and for providing supporting documentation for validation and submission to regulatory authorities.
By assessing other factors such as process costs, operational flexibility, sterility assurance, and utilities, you can make an informed decision on whether single-use bioreactors such as the Allegro STR 200 unit provide significant process improvements in mammalian cell culture and offer a beneficial alternative to stainless steel, cleanable systems.
References
1 Rader RA, Langer ES. Upstream Single-Use Bioprocessing Systems. BioProcess Int. 10(2) 2012: 12–18.
2 Isailovic B, Kradolfer M, Rees B. Fluid Dynamics of a Single-Use Stirred Tank Bioreactor for Mammalian Cell Culture. BioProcess Int. 13(8) 2015: 60–66.
3 Pall Publication USD2923: Allegro STR 200 Single-Use Stirred Tank Bioreactor. Pall Life Sciences: Port Washington, NY.
4 Pall Publication USD2926: Cultivation of CHO cells in Allegro™ STR 200 Single-Use Stirred Tank Bioreactor System. Pall Life Sciences: Port Washington, NY.
5 Godoy-Silva R, et al. Physiological Responses of CHO Cells to Repetitive Hydrodynamic Stress. Biotechnol. Bioeng. 103(6) 2009: 1103–1117.
6 Gray DR, et al. CO2 in Large-Scale and High-Density CHO Cell Perfusion Culture. Cytotechnol. 22, 1996: 65–78.
Bojan Isailovic is principal R&D engineer, Byron Rees is senior R&D manager for cell culture applications, and Alison West is director, R&D design engineering, all at Pall Life Sciences, 5 Harbourgate Business Park, Southampton Road, Portsmouth, UK.
You May Also Like





