Development of a Novel Cell-Separation Platform: Discussion with Quad Technologies CEO Sean Kevlahan
April 18, 2016
Releasing and separating cells from surfaces and capture molecules are critical steps in cell therapy development. Research into such therapies as chimeric antigen receptor T cells (CAR-T) cancer therapies and stem-cell regenerative medicines demand the isolation and purification of viable and functional target cells. A number of cell-separation strategies can be used to produce such cells, but they are not able to deliver the required efficiency or scalability and can also cause damage to cells or affect their phenotype.
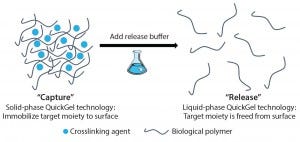
Figure 1: QuickGel hydrogel platform
Since its foundation in 2013, Quad Technologies has focused on its platform QuickGel technology, a dissolvable “cell friendly” hydrogel (Figure 1). In late 2015, the company also launched its MagCloudz streptavidin cell-separation kit and partnered with the University of Massachusetts Medical School in research toward the enrichment and purification of CD3+ T cells from human umbilical cord blood. The collaboration is part of the company’s overall plans to find new partnerships “focused on technology development and refinement for applications critical to regenerative medicine and immunotherapeutic areas.” BPI spoke with CEO and cofounder Sean Kevlahan, PhD, to gain his perspective on the advancements of the technology and how it fits the needs of the industry.
Foundations
How did Quad Technologies and its QuickGel platform start? What needs does this technology address in the cell therapy and regenerative medicine industries? QuickGel was first developed at Northeastern University, where I did my thesis work. We developed a surface chemistry that enables the capture and release of cells off of substrates without deleterious effects. There are many ways to release cells, but it can be difficult to keep those cells in tact when you release from substrates. So that is what we focused on achieving at Northeastern. We saw a long of commercial interest from people coming to our laboratory and saying how cool the chemistry was and how broadly applicable it was.
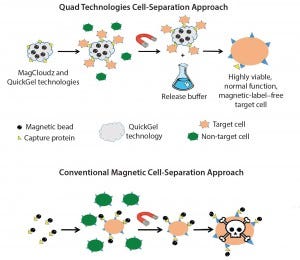
Figure 2: MagCloudz cell separation (top) uses magnetic particles as a carrier for the QuickGel technology. Target cells are captured through magnetic cell isolation workflow. After cell separation, MagCloudz system is dissolved to release target cells from magnetic carriers, which can then be removed to leave the cells in their natural state. By contrast, conventional magnetic-separation– based strategies (bottom) can retain magnetic labels.
I have always had an entrepreneurial spirit. So with the support of investors, I took the technology out of Northeastern and formed Quad Technologies in 2013. We have focused on commercializing the QuickGel chemistry and its applications within the broad cell-processing world. Our first application in the research market includes QuickGel hydrogel applied to magnetic particles in the MagCloudz cell separation kit (Figure 2). In the kit, we tether capture proteins to QuickGel hydrogel and perform cell purification. At the end of the cell-isolation process, end users decouple the magnetic particles from target cells with a release buffer and clear magnetic label contaminants.
Are these developments for commercial-scale applications? The kits are currently for research-use-only. We are currently developing clinical good manufacturing practice (GMP) grade QuickGel hydrogel.
State of an Industry
How do these technologies fill the needs of the current cell therapy industry? Within bioprocessing and cell bioprocessing applications, guaranteeing the integrity of your product is critical. That’s where a lot of the “pain points” for end users exist. For many CAR-T and T-cell receptor (TCR) cell therapies, there’s a significant need to scale-up processes. However, problems often occur downstream for such processes. So how do you get a defined cell population at the end of your process while keeping a product intact and alive so that it can be used robustly?
After seeking feedback from researchers working in the industry, there are frustrations around the limitations of antiquated technologies that have been around since the 1990s. Companies are looking for ways to take their bioprocesses to the next level, and downstream purification of their cells is a critical component of that. That’s where our QuickGel technology comes in. We have the potential to enable our chemistry onto much larger formats, so users can capture and enrich cells. After purification of the cells that are still stuck on the substrate, you can release those cells, while maintaining essential viability.
What are some of the industry’s current problems with cell viability and recovery? If you look at current technology workflows today that are in clinical bioprocessing, companies are seeing recoveries of at most, maybe about 50% of target population. So that means about 50% of product is thrown away. And their viabilities are approximately 85% or a slightly higher. For rare cell populations, these losses in recovery and viability present challenges for end users performing research on a process with potential for transfer to clinical scale by decreasing the ability for process time and cost efficiency. Such challenges are directly related to the high cost and broad availability of patient therapies we see today. Limited technology solutions currently exist to truly overcome those hurdles, so there are other costly business decisions for the industry to make regarding developing these solutions themselves or bringing in and adapting existing technologies from external sources.
What other trends are you seeing in this area? The overarching trend right now in bioprocessing and pharmaceuticals is essentially the shift from small molecules and biologics to now cell and gene therapies. There has been a significant trend in biotechnology with companies investing in different cell therapy workflows. That has been in part a result of how much response efficacy that has been achieved with some patients in addition to work with different types of gene therapy applications and coupling those with cell therapy advancements.
Overall, there is significant movement in the biotechnology and pharmaceutical space. For bioprocessing and processing in general, these organizations are still trying to catch up with the latest developments in research and development. That is why we are developing the QuickGel technology: to truly enable rapid development into a clinical bioprocess. Our chemistry is differentiated in unique ways that allow users from the research-only workflow to bring our chemistry to a larger-scale bioprocessing format.
Applications and Future Development
What kind of processes and cell types would be appropriate to use with the QuickGel platform? It can be used on any cell process as long as there’s an extracellular marker that it can grab onto. The platform chemistry is agnostic to the cells that it gets isolated against, and then chemistry is flexible, which allows either purification of T-cells, hematopoietic stem cells, or NK cells, for example.
What progress is still needed for the cell therapy and regenerative medicines segments of the industry to achieve commercial success? One of the most limiting steps at the moment is cost of goods, specifically the cost of intracell therapies and process time efficiency. Scale up and downstream purification are other limiting steps.
In your workshop at the Cell and Gene Therapy World conference earlier this year, you showed a closed and automated process. It looked like you’re addressing what everybody is talking about: that these therapies were not going to make it to commercial viability unless they can reduce manual intervention. Would you give us a quick overview of how your technology, as an example, is addressing this? Running a closed system is one of the big issues that a lot of end users are looking at today. Because Quad Technologies has a platform chemistry that can couple into those closed systems, we’re finding and have identified some potential partners within the industry. With those collaborations, our goal is to develop and eventually integrate the QuickGel platform into these closed cell therapy systems, allowing us to meet the scale and efficiency demands of the clinical bioprocess.
What are your plans going forward? Our plan is to continue product and technology development and eventually offer a GMP-grade QuickGel product. Our hope is that we’ll register QuickGel with the FDA Master Device file. In that way, people can cross-reference their investigational new drugs (INDs) with our chemistry, and they can feel comfortable that they have a viable processing workflow.
Maribel Rios is managing editor of BioProcess International; mrios@ bioprocessintl.com. All quotes and figures were obtained from personal communication or from http://quadtechnologies.com.
You May Also Like





