From Interest in Intensification to a Factory of the Future
Much has been published on improvements and advances in many individual technologies for biomanufacturing. If you take a comprehensive look at the field, however, you find overlap, muddling, and even contradiction about which particular processes or aspects of technological development should be designated properly as process intensification. Although the industry is addressing such distinct goals as improved manufacturing yield, product quality, and cost-effectiveness, the names of initiatives commonly applied to accomplish those goals overlap at best. Such ambiguity and lack of precision can lead to inefficiencies and errors across the industry or even within individual companies (1).
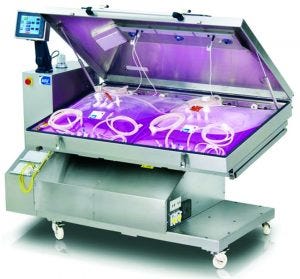
Perfusion can improve upstream productivity in a number of ways. When used in seed trains, it can increase temporal and footprint productivity. When used in production, it can increase volumetric productivity. Also shown here is a single-use ReadyToProcess WAVE Cellbag bioreactor from GE Healthcare.
The Cambridge University Press dictionary defines intensification as “the fact of becoming greater, more serious, or more extreme, or of making something do this.” For modern industries in general, process intensification has been defined as “any chemical engineering development that leads to a substantially smaller, cleaner, safer, and more energy efficient technology” (2). But based on that definition, most improvements of any sort in a biomanufacturing process might be termed “intensification,” and some authors have been using the word in such a way.
However, many experts in the biomanufacturing community use the phrase process intensification only when referring to an improvement in the productivity or economy of a process, such as to “take an existing process and optimize it to increase output: more product in a shorter time, with fewer steps, and from a smaller working footprint” (3). Acceptance of such usage would indicate that, for example, a process simplification or a method of reducing an undesirable product glycan may be desirable, but wouldn’t be considered an “intensification” of the process. We suggest that the biopharmaceutical industry might consider clarifying this issue by establishing an industry standard for such biomanufacturing terms, as has been accomplished for many others in ISPE’s glossary of pharmaceutical and biotechnology terminology (4). Also, an ASTM committee on manufacture of pharmaceutical and biopharmaceutical products has addressed the development of standardized nomenclature and definitions of terms (5).
Here we delineate and define a number of process improvement goals for upstream biomanufacturing, to identify technologies and equipment proposed or recently implemented to accomplish those goals, and to exemplify which of those initiatives would improve manufacturing productivity and thus truly intensify bioprocesses.
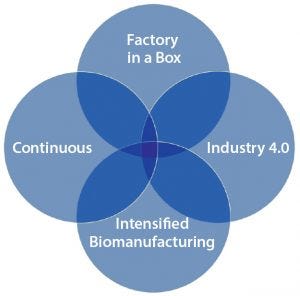
Figure 1: Constituents of “factory of the future” or “smart bioprocessing” — their goals, technological bases, and applications can overlap to varying degree.
New Directions
The next-generation “factory of the future” and “smart bioprocessing” designators (as often used) include many distinct and recently initiated technologies (Figure 1). As you might expect, they include developments in digital biomanufacturing components such as big data and process automation (6). In fact, we are seeing remarkable innovations in nearly all biopharmaceutical entities, processes, platforms, modes, equipment, materials, and facilities. It’s important to note that
many developing technologies apply to more than one process improvement goal
many individual process development groups are focusing on different applications or end goals from each technology.
For example, a media supplement might increase the volumetric productivity of a bioreactor operation even if it was designed for a different primary application (e.g., improving product quality). So it may be more efficient and clearer to use process intensification only when referring to developments that are intended to provide increased productivity of some type, such as the many perfusion applications do. We suggest maintaining the designators “factory of the future” and “smart bioprocessing” as phrases to include technologies that support all sorts of process and product improvements (Table 1). However, which particular terms are restricted in their scope, and which are allowed to remain more comprehensive, is not as important as is codifying in some way the decisions made.
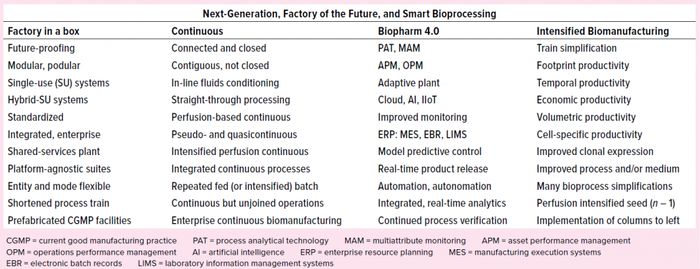
Table 1: Many individual initiatives are revolutionizing biomanufacturing. Having arrived from different scientific and engineering disciplines, they relate to each other in unique ways. This table attempts to identify and organize some major programs and technologies currently implemented in upstream biomanufacturing. Many programs support or otherwise closely relate to each other in some essential way or have been initiated in another context and now are being applied to biomanufacturing. Some have been described and defined in literature by authors of disparate backgrounds, thus the same initiatives have been named and/or described using different terminology. Many terms have been used to refer to different groupings or organizations of elements from the same overall set of component technologies.
Under the current (sometimes very broad) usage, precisely what aspects of biomanufacturing have been improved when the phrase process intensification is applied include facilities, equipment, and process control; mass transfer and mixing; capital costs, energy use and process footprint; consolidation of processing steps; and reduction in suite classification and environmental stress. The many goals often considered in this approach therefore include making a process cleaner, cheaper, smaller, more efficient, safer, and faster than it was before. In biomanufacturing, that could include higher cell densities, manufacturing flexibilities, sustainability considerations, improvements to product quality, and more. There is little to no improvement in a material, process, or equipment that hasn’t been regarded by someone to be “part of an intensification approach.”
On the other hand, as we introduced above, many experts emphasize activities that increase only one dimension of a process step: yield (7). A recent article on process intensification for the biopharmaceutical industry clearly states, “Process intensification is used to get more out of the process, whether it’s by producing more product upstream or retaining more product downstream” (3). Yet another defines bioprocess intensification in different terms but to the same conclusion as “smaller footprint/volume” systems that “yield the same desired results and performance” (8).
In any of the descriptions above, the scope of process intensification ranges from unit operations to entire processes and even extends to design of entire facilities and construction approaches. An increasing scope directly correlates to the degree of organizational impact and subsequent effort required for successful implementation, which underscores the importance of terminology during communication for goal-setting and alignment. However, biomanufacturers increasingly find the benefits — flexible manufacturing, future-proofing, modality agnostic suites, and so on — to be well worth the extra effort.
For example, “factory in a box” as used herein refers to prefabricated facilities optimized at every level of biomanufacturing: process, capacity, equipment, automation, and human competencies (training). In one example a biomanufacturer cut factory construction time to 18 months from three years, reducing costs by 25–50% compared with traditional facilities, reducing CO2 emissions by 75%, and cutting water and energy use by 80% (9). Prefabricated, predesigned facility concepts (e.g., modular, podular, and ballroom/dancefloor) integrate and extensively leverage the single-use technologies and intensified processes discussed here, even using hybrid formats.
Intensified Biomanufacturing
Along with the discussion above, we have considered the body of published literature and the many developing technologies and categories of product and process improvement ongoing today to propose a definition of intensified biomanufacturing as “technologies to increase the amount of intermediate or final product manufactured per volume, footprint, unit of time, or expense by increasing efficiency in one or more unit operations.” We see bioprocess intensification as a means to accomplishing that — to the extent that it is basically a synonym for it. And we believe that such technologies may be realized using a few distinct materials or types of equipment (e.g., pictured on the first page of this article). This is not meant to diminish the novelty or importance of such exciting initiatives as digital recordkeeping, novel manufacturing execution and enterprise resource planning systems (MES/ERP), advances in product-quality monitoring, or assurance of raw material supply and quality. We intend rather to establish better communication through an economy and limitation of topics to address under this particular heading.
Even if we constrain process intensifications as approaches to increasing some aspect of productivity in a biomanufacturing facility, a number of distinct technologies are emerging to support this concept. They range from increasing the specific productivity of a platform cell-line to improvements in a facility, process, operation, or materials. For example, one way to increase temporal and footprint productivity is through an intensified seed train by cryopreservation of more cells in each aliquot of working stock. Rather than freezing concentrated cell suspensions in portions of 1 mL to 5 mL, techniques have been developed to support volumes up to multiple liters. Having more cells with which to seed a culture allows a production process to begin in larger containers or bioreactors, which can save one or more cycles of culture initiation. Moving from seed directly to an n – 1 reactor has become possible in many operations.
Perfusion Culture
The fed-batch culture mode is the mainstay of current biomanufacturing. Essentially it involves midoperation supply of nutrients (substrates and/or primary metabolites) to a bioreactor in
support of relatively high cell densities, product accumulation, or both. Typically it is followed by harvest of the entire culture. There are a number of variations on the basic idea (10–13), from adding a bolus of a designed nutrient mixture at previously identified points in the process to parametrically determined periodic or continuous feeding with the composition and/or volume varied according to feedback from near–real-time monitoring (termed feed-forward control).
However, perfusion culture is gaining attraction as a means to support a few distinct values. Perfusion has an interesting history in biomanufacturing because of one if it’s many capabilities: It’s serving as a means of continuous bioreactor operation. Decades ago, this approach appeared promising for the production of many products. Through different mechanical means, equivalent volumes of media would be added and removed simultaneously, with (some) cells retained in the bioreactor. About 20 approved products have been manufactured in this way (14).
Perfusion has maintained very limited application in biomanufacturing because of constraints on the equipment to segregate cells from media, the ability to monitor the status of a reactor, and available technology to control the process. However, increased understanding in those areas and of the requirements of cultured cells have led to implementations of perfusion technology in a number of process formats. Intensified perfusion in particular has become popular. It is established by retaining more cells in a bioreactor during perfusion to amplify cell density to a predefined level – followed either by harvest (an intensified fed batch) or nearly steady-state operation (what’s referred to as continuous biomanufacturing).
Intensified perfusion presents another way of accelerating seed-train processes, as mentioned above. It can be used to increase the concentration of cells in n – x reactors and therefore accelerate progression to a main production bioreactor. As with increasing the amount of cryopreserved seed, this sometimes can support the use of only one bioreactor in train.
Repeated intensified fed-batch culture is another use for perfusion that has been receiving interest of late. The periodic harvesting of most material from a bioreactor culture (which becomes the first batch) and replenishing media to allow for subsequent culture expansion provides benefits such as elimination of seed trains and bioreactor preparation for subsequent batches. Using perfusion here to amplify cell (and) product concentrations greatly intensifies such processes as well.
Increased Productivity
Productivity can be measured in many different ways. The biopharmaceutical industry has identified incremental accumulations of product by time, footprint, volume, or investment (cost). Both the temporal and footprint productivity of a plant can be improved by implementing single-use technologies, which greatly reduce turnaround times between batches and the space that otherwise would be required for extensive steam and water facilities for cleaning reusable equipment. Beyond increases in volumetric productivity outlined above, perfusion culture also can increase temporal and footprint productivity by shorting the duration of seed trains; by eliminating delays associated with batch initiation, harvest, and turn-around; and by reducing both bioreactor size and the number of early days relegated to less efficient, low cell-density operations.
A number of technologies can contribute to volumetric productivity. For example, improvements in recombination vector technologies, clone generation and selection approaches (as well as in culture media design) can improve cell-specific productivity of a platform clone, thereby increasing the amount of product generated per volume of culture at a nominal culture density. Supporting systems here include site-specific gene modification through zinc-finger nucleases and clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated protein 9 (Cas9) technology. Five times the amount of product secreted per cell can yield five times the amount of product produced per liter of culture. But intensified perfusion of clones with unmodified cell-specific productivity will increase volumetric productivity simply by increasing the number of cells per volume of culture. So five times the number of cells per liter could yield five times the amount of product produced per liter.
Table 1 lists a number of new developments that are not categorized under the Intensified Biomanufacturing column but support other goals and can nonetheless improve productivity in certain implementations. For example, many Industry 4.0 technologies can help reduce process failures, ultimately resulting in increased temporal productivity. Incorporating automation on the manufacturing floor can reduce the labor required for each batch and thus increase economic productivity. The economic productivity of a process can be improved by simplifying that process. For example, streamlining, automating, or outsourcing the handling of raw materials or preparation of growth media and buffers can improve the economic productivity of a manufacturing facility by reducing the cost of preparation for each batch it produces.
Advanced process monitoring and control can support extremely intensified perfusion through robust bioreactor operations (>100 × 106 cells/L), resulting in greatly increased volumetric productivity. Judicious application of process analytical technology (PAT) and multiattribute methods (MAMs) in process development can help provide better-producing clones and more efficient culture media, process supplements, or control algorithms, thus yielding processes that display increased performance and yield. ERP as enabled by MES, electronic batch records (EBRs), and laboratory information management systems (LIMS) can improve the temporal productivity of a process by preventing delays and failures in scheduled runs.
Modularity in biomanufacturing arises from many distinct and standardized instruments, assemblies, or facility components that can be assembled in different configurations and/or locations. In general, it requires standardized components that can be arranged (and rearranged) in multiple ways over a long period, thereby affording significant flexibility and scalability. We find it interesting that modularity is yet another ambiguous term. Architect Eric Bohn has described modular construction as “a term used indiscriminately to describe many unrelated approaches ranging from process/utility skids and preengineered, modular cleanroom wall systems up to the prefabrication of entire buildings” (15).
Modular and podular facilities can increase the temporal productivity of a company by making plants process or product “agnostic” (to borrow an information technology term): generalized so that it is interoperable among many different systems. That can provide a number of benefits such as reducing changeover time. Open floorplans — including those with preengineered cleanroom wall systems, modular unit operations, and ceiling drops for utilities — can do the same (while providing other potential values). Skid-mounted modular equipment can save installation and validation time through movability, allowing transfer to individual product manufacturing suites on demand or even from process development to operations either to replace defective equipment or reduce technology transfer time.
Such innovations as “plug and play” equipment not only reduce the time needed to reconfigure a suite or process train, but they also can save time in scale-out–based production expansion initiatives because they can be replicated readily in new suites or facilities — even in other countries. For many applications, process expansion and replication through modular devices also reduces validation/qualification costs. Examples of such flexible manufacturing approaches include GE Healthcare’s FlexFactory system for dynamic orchestration of unit operations and KUbio in modular facility design.
Why Intensification?
Goals in considering intensification technologies include increases in return on assets/investment (RoA/RoI) and plant or process productivity and reductions in infrastructure planning, construction costs and duration, project timelines, and failure risk. All change comes with the risk of unintended collateral consequences, however. Therefore, other factors to consider when you examine available options include maintaining product quality, regulatory compliance, manufacturing agility, and employee satisfaction.
Cost sensitivity and process economics often drive decision making to a degree that is equal to (if not greater than) technical solution readiness or efficacy. With many options of different maturities available, taking one intensification approach can lead a company quickly to a point of relative diminishing return. Conducting a needsbased analysis for specific process settings is the recommended way of approaching a decision (16). Such analyses can elucidate the point in a process at which an intensification strategy will produce desired positive effects while helping to catalogue possible secondary or hidden consequences.
For example, the goals and tactics of a strategic process improvement team pursuing generally forward-looking technologies as a means to reduce operating costs or future-proof an established production platform would be markedly different from those of a team focusing on bringing a new molecule to market with an identified progressive process. The latter team must remain aware of such pressures as “speed to market,” impending clinical trials requiring drug-product material, and such options as pursuing internal or outsourced manufacturing options.
Finally, the sustainability of manufacturing processes is of growing concern to suppliers, manufacturers, and consumers alike. Increasing productivity in most metrics implies that more product will be manufactured with the same materials, equipment, or facility. Because all of those impose an environmental burden of some sort, it is likely that more intensified processes will reduce the impact of biomanufacturing on the environment.
A Defining Moment
Biomanufacturing is benefiting from many new technologies and innovations. Some suppliers use language such as in the headings of Table 2 for referring to the same set of initiatives. We suggest establishing a definition of process intensification that refers to an increase in product yield as the primary intent. The biopharmaceutical supply industry might consider establishing some accepted glossary or lexicon to ensure that everyone is using the same vocabulary to illustrate a problem to be solved, options available, and reasoning behind the path chosen.
References
1 Ratz D. Terminological Consistency: Quality Through Precision. Amplexor: Luxembourg, 7 April 2017; https://blog. amplexor.com/globalcontent/en/ terminological-consistency-qualitythrough-precision.
2 Joyce PC. What Is Process Intensification and How Can You Implement It? Epic Systems: St. Louis, MO, 2019; https:// www.epicmodularprocess.com/blog/what-isprocess- intensification.
3 Smith SF. Process Intensification: Getting More From Less. Medicine Maker 17 January 2019; https://themedicinemaker. com/manufacture/process-intensificationgetting- more-from-less.
4 Glossary. ISPE: North Bethesda, MD, 2019; https://ispe.org/glossary.
5 Committee E55 on Manufacture of Pharmaceutical and Biopharmaceutical Products. American Society for Testing and Materials: West Conshohocken, PA; https:// www.astm.org/COMMIT/SCOPES/E55.htm.
6 Whitford W. The Era of Digital Biomanufacturing. BioProcess Int. 15(3) 2017: 12–18.
7 Continuous Biopharmaceutical Manufacturing: Can It Live Up to the Hype? iSpeak Blog 4 January 2017; https://ispe.org/ pharmaceutical-engineering/ispeak/ continuous-biopharmaceuticalmanufacturing- can-it-live-hype.
8 Montgomery SA, Scott C, Rios M. Chapter 8: The Manufacturing Perspective — Current Approaches to Bioprocess Intensification. BioProcess Int. 15(6) 2017: ebook.
9 Egan M. Think Inside the Box: Pfizer Will Use GE’s Mobile Biotech Factory to Make Next-Generation Drugs in China. GE Reports 30 June 2016; https://www.ge.com/reports/ pfizer-will-use-ges-modular-factory-to-makenext- generation-drugs-in-china.
10 Yang WC, et al. Perfusion Intensified Seed Cultures Supporting High-Density Seed Production. Biotechnol. Prog. 30(3) 2014: 616–625; https://www.ncbi.nlm.nih.gov/ pubmed/24574326.
11 Zhang A, et al. Advanced Process Monitoring and Feedback Control. Biotechnol. Bioeng. 112(12) 2015: 2495–2504; https:// onlinelibrary.wiley.com/doi/pdf/10.1002/ bit.25684.
12 Walker N. Perfusion Appears to be Gaining Traction in Biopharma Manufacturing. Pharma’s Almanac 14 September 2017; https://www. pharmasalmanac.com/articles/perfusionappears- to-be-gaining-traction-in-biopharmamanufacturing.
13 Pollock J, et al. Integrated, Continuous Biomanufacturing: Economic, Operational, and Environmental Feasibility for Clinical and Commercial Antibody Manufacture. Biotechnol. Prog. 33(4) 2017: 854–866; https://www.ncbi.nlm.nih.gov/pmc/articles/ PMC5575510.
14 Schmidt SR. Drivers, Opportunities, and Limits of Continuous Processing. BioProcess Int. 15(3) 2017: 30–37; https:// bioprocessintl.com/manufacturing/ continuous-bioprocessing/driversopportunities- limits-continuousprocessing/?_ sm_au_=i0HWPtVtjDHbk66s.
15 Bohn E. Deciding When to Use Modular Construction. Pharm. Technol. 40(6) 2016: 45–46.
16 Galliher P, Jagschies G, Dua AR. Continuous Bioprocessing: Is It for Everyone? Gen. Eng. News 37(16) 2017; https://www.genengnews.com/magazine/300/supplementcontinuous- bioprocessing-is-it-for-everyone.
Corresponding author and BPI editorial advisor William Whitford is strategic solutions leader for upstream at GE Healthcare, Logan, UT; 1-435-757- 1022; [email protected]. Daniel Nelson is senior strategic marketing leader in upstream at GE Healthcare, Marlborough, MA; 1-435-757-0286.
You May Also Like





