Designing the Right Strategy for Digital Transformation: How a Pragmatic Approach to Digital Transformation Can Help Biomanufacturers Adapt to a Challenging Future

Figure 1: Database of high-value use cases segmented by value opportunity with key information on each
(QC = quality control)
Although the biopharmaceutical industry has enjoyed explosive growth over the past three decades, it still faces an assortment of challenges. Those include growing portfolio complexities, increased demand volatility, stringent regulatory requirements, increased pricing pressures, and growing technological complexities, all leading to severe pressure on profit margins. To overcome such pressures, biopharmaceutical operations need to become more reliable and agile, and they must realize efficiency gains in both manufacturing and supply chains.
Digital transformation offers strong value opportunities, including a potential for reducing 10–20% of typical conversion costs. Given both the wealth of data available in biotechnology facilities and the resources and capability to invest, biopharmaceutical companies should be well-positioned to benefit from such a transformation, but they experience many of the same challenges as companies in other industries. In many ways, the biopharmaceutical industry lags behind other industries such as media, retail, and automotive in delivering value and results from its digital journey. Below, we highlight six common challenges that biopharmaceutical companies face.
Absence of a Cohesive Strategy and Roadmap for Digital Transformation: Many companies lack the requisite strong, organization-wide roadmap, so they face a number of potential problems. Digital initiatives often are siloed and uncoordinated, which can lead to duplicated efforts, reduced impact, and an inability to change the overall course of an organization. In some cases, project managers from a company’s information technology (IT) function will run the show — rather than business owners (often end customers of digital solutions) — leading a company to lack both an understanding of gaps in its business processes and a plan for addressing them. Some biomanufacturers attempt a “big bang” approach to digital transformation: aiming to prevent siloes or sidelining of the effort by mandating an adoption of a certain tool or technology. But within a few months, many of those companies watch their initiatives get bogged down in delays and overruns. Another common mistake is to create an “ivory tower.”
Companies concentrate digital experts at the corporate center rather than building capabilities within the business and restructuring processes to maximize results.
Without a well–thought-through strategy and a roadmap that is clearly communicated to all in an organization, a lack of direction, a shortage of capabilities, and poor crossfunctional alignment are likely to develop.
Lack of Focus on High-Value Use Cases: Too often, biomanufacturers have neither a rigorous value focus for their digital transformation nor a clear view of all the use cases and technologies that can be implemented to deliver maximum value for their companies. Some facilities will implement technologies, feeling that they “ought” to invest in digital tools, but then fail to channel them toward their companies’ greatest value opportunities. Other biomanufacturers will identify value opportunities but fail to recognize digital technologies as part of a solution to a problem.
Difficulty for Leadership to Understand Technologies and Envision Potential Impact: Digital transformation has the greatest impact when implemented throughout an organization. However, it is unrealistic to expect executives to stay up to date with rapidly changing innovations that could drive value for their businesses. Thus, many of them struggle to provide an organization-wide effort as well as needed resources and support.
Lack of Investment in Digital Capability: Ultimately, biomanufacturing employees are needed to implement digital tools and maximize their value. Too often, companies default to a system-centric approach that prioritizes system selection and assumes value will come from implementation alone. A much higher likelihood of success will come from a people-centric approach that identifies and builds talent and then empowers subject-matter experts (SMEs) in operations to implement use cases that deliver value.
Difficulty Piloting Experimental Technology: Controls on good manufacturing practice (GMP) facilities and pressures to deliver commercial production volumes in highly used plants often make it difficult for companies to pilot innovative technologies. Trying out a new digital technology requires a certain degree of confidence, but until a trial has been completed, getting required buy-in can be difficult.
Lack of Regulatory Clarity Relating to Use of Emerging Technologies: Lack of consensus tends to add complexity to an already challenging decision process on implementing technology in a GMP environment. Many technologies, particularly those that move toward a “bionic” organization in which decision-making shifts from humans to technology, have not yet entered the mainstream of what is considered routinely acceptable to regulators.
Solutions to Enable Digital Improvements
Overcoming those problems starts with a clear focus on a digital transformation’s potential to make a whole organization more efficient and competitive rather than isolated parts of it. Senior leaders must adopt a clearly articulated digital vision, strategy, and roadmap. That creates a foundation to identify “lighthouse” use cases that can help prove the value of a digital transformation and build momentum for wider adoption.
Selecting the right use cases at the outset, focusing on delivering value, and ensuring that the right capabilities are in place all are prerequisites to building momentum in a digital transformation. That momentum can be leveraged to accelerate digital-capability building at scale and to industrialize successful pilots.
As part of the Biopharma 4.0 (B4.0) Alliance, Boston Consulting Group (BCG) and Ireland’s National Institute for Bioprocessing Research and Training (NIBRT) recently established the Innovation Center for Operations (ICO) in Dublin.
The B4.0 Alliance aims to help biopharmaceutical manufacturers develop their own “factories of the future,” by delivering sustainable performance improvements across their networks. Together, BCG and NIBRT are leveraging different ICO offerings to help biopharmaceutical companies overcome many of the issues identified above.
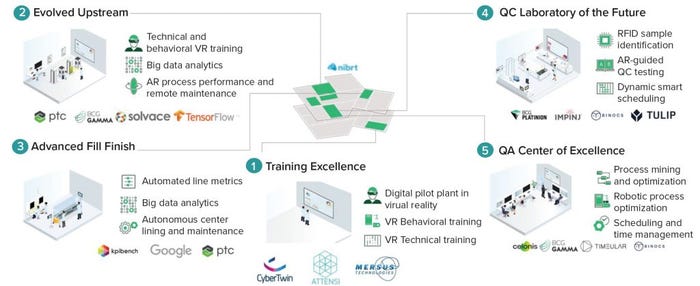
Figure 2: The Biopharma 4.0 Innovation Center (QC = quality control, QA = quality assurance, VR = virtual reality,
AR = augmented reality, RFID = radiofrequency identification)
Technology Immersions: To acquaint senior executives, digital leaders, and operational SMEs with the understanding and inspiration they need to help them develop a vision for their companies’ digital transformations, the ICO provides an immersive tour across five digital workstations (Figure 2). Visiting teams can discover the full range of Industry 4.0 technologies — demonstrated in a GMP environment and inspired by biopharmaceutical-specific digital-use cases. The five areas include
training excellence, including digital pilot-plant, behavioral training, and technical training, all in virtual reality (VR)
evolved upstream, including technical and behavioral training through VR, big data analytics, augmented reality (AR), and remote maintenance
advanced fill–finish, including automated line metrics, big data analytics, and autonomous center-lining and maintenance
a quality control (QC) laboratory of the future, covering RFID simple identification, AR-guided QC testing, and dynamic smart scheduling
a quality assurance (QA) center of excellence, covering process mining and organization, robotic process optimization and scheduling, and time management.
Strategy and Roadmap Development: Inspired by technology demonstrations in the ICO and a clear idea of the “art of the possible,” senior leaders can develop a clear strategic vision and a proposed roadmap based on appropriate use cases needed to achieve their own visions. A biomanufacturer then can work with ICO experts to create a program and devise solutions that deliver immediate impact and build momentum.
Use-Case Prioritization Sessions: Digital transformation teams and operational SMEs can participate in workshops leveraging a database of high-value use cases (Figure 1). The database is indexed to enable users to select and prioritize use cases that apply most directly to their organizations and objectives.
Digital Capability Building: Personnel development is critical to implementing digital transformations at scale and driving continuous improvements. The ICO provides training for digital leaders, operational SMEs, data scientists, and other stakeholders who will be implementing digital transformations. This training is structured around three pillars:
digital technology and immersion training on specific technologies (e.g., advanced analytics and AI, AR/VR, and Industrial Internet of Things)
work-center–focused case studies, including trainee-specific operational situations across fill–finish, packaging, QA, QC, and upstream/active pharmaceutical ingredients (APIs)
the immersive sessions in each of the five digital workstations (Figure 2).
Experimental Technology Piloting: The ICO creates a safe, immersive GMP environment for piloting and testing technologies, outside a company’s own commercial sites, thereby eliminating risk to ongoing commercial production.
Bringing Regulatory Clarity: The ICO is building a robust common understanding of regulatory implications of such technologies and use cases (e.g., testing sterility of an AR headset). By inviting regulators to tour the ICO and understand I4.0 technologies, the ICO will lead the two-way conversation on aligning regulatory and commercial interests.
The Way Ahead
An increasingly challenging landscape — including rising costs, demand volatility, manufacturing process complexities, and an intensified regulatory environment — is forcing the biopharmaceutical industry to rethink its traditional models. Digital initiatives hold the key to overcoming many current pressures and challenges. However, a lack of pace, value focus, and leadership support of digital transformation efforts is limiting success. Companies should review their digital strategies, including the resources and talents needed, and focus on value to start on the road to transformation.
James Morton is an associate director in Boston Consulting Group’s London office, specializing in biopharmaceutical operations. Killian O’Driscoll is director of projects at NIBRT.
You May Also Like





