Facing a Unique Challenge: Building an In-House Cell and Gene Therapy Manufacturing Facility During the Pandemic
 In 2019, Expression Therapeutics (ET) obtained investigational new drug (IND) approval for its lead clinical product. The third-generation lentiviral vector (LV) expresses a bioengineered coagulation factor VIII to be used in an autologous hematopoietic stem- and progenitor-cell gene therapy for patients with severe hemophilia A. Like many other emerging biotechnology companies, ET’s initial strategy used reputable contract development and manufacturing organizations (CDMOs) for vector and cell manufacturing needs and a prominent clinical contract research organization (CRO) with extensive experience in early cell and gene therapy trials to support clinical development. However, we revisited the decision to do so based on our early experience and knowledge of discrepancies encountered by others in transitions from CDMO to internal manufacturing between phase 1–2 and phase 3. Challenges encountered in outsourcing included delays in securing viral vector manufacturing slots caused by capacity shortfalls, lack of flexibility for accommodation of minor process or product changes, challenging technology transfer, and delayed contract negotiations.
In 2019, Expression Therapeutics (ET) obtained investigational new drug (IND) approval for its lead clinical product. The third-generation lentiviral vector (LV) expresses a bioengineered coagulation factor VIII to be used in an autologous hematopoietic stem- and progenitor-cell gene therapy for patients with severe hemophilia A. Like many other emerging biotechnology companies, ET’s initial strategy used reputable contract development and manufacturing organizations (CDMOs) for vector and cell manufacturing needs and a prominent clinical contract research organization (CRO) with extensive experience in early cell and gene therapy trials to support clinical development. However, we revisited the decision to do so based on our early experience and knowledge of discrepancies encountered by others in transitions from CDMO to internal manufacturing between phase 1–2 and phase 3. Challenges encountered in outsourcing included delays in securing viral vector manufacturing slots caused by capacity shortfalls, lack of flexibility for accommodation of minor process or product changes, challenging technology transfer, and delayed contract negotiations.
ET’s rich and diverse therapeutic pipeline includes novel gene therapies for hemophilia A/B, neuroblastoma (NB), and leukemia/lymphoma. With a desire to move each of those candidates into clinical development as early as possible, management considered a strategic pivot to bring in-house manufacturing online. Such a strategic change would give ET internal and direct control over all clinical development efforts. Providing end-to-end solutions, that intended support would cover material availability across all stages, including research and development (R&D), proof of concept, preclinical testing, early clinical studies, late-phase development, and eventual commercialization.
To develop all assets internally as a fully integrated cell and gene therapy company, ET would need to build its own manufacturing facility. This pivot required hiring an experienced chief executive officer (CEO), increasing staffing from a few personnel to more than 30 people (with medical and other doctorate-level expertise), and transfer of a current good manufacturing practice (CGMP) viral vector manufacturing team along with quality assurance (QA) personnel from a regional vector manufacturing organization. We viewed the GMP team as mission critical because very few such teams can be found, especially those like ours who have experience with more than 75 products (several used in late-stage clinical trials by large pharmaceutical clients). Such a team could hit the ground running after facility build-out. That project would include all traditional activities such as site selection, identification of an architectural and engineering group, choice of what type of cleanrooms to install, hiring of a project-management team, and securing an experienced construction firm. Such activities should be followed seamlessly by commissioning and validation of a facility, its equipment, and the processes to be housed.
Even under ideal circumstances, the requirements and resources needed to build, equip, qualify, staff, and operate a modern cell and gene manufacturing facility are complex. However, even as ET was making these decisions, a novel coronavirus was about to emerge into a global pandemic that would alter the entire biopharmaceutical industry fundamentally as it affected the whole world. Building during the pandemic would require a paradigm shift in how to approach management of personnel, resources, time, and supply chains — not only for construction materials, but also for equipment and raw materials. During this project, neither ET nor any members of the professional team of engineers, contractors, and personnel experienced any COVID-19 outbreaks or lost days due to such illness. Furthermore, the project was completed both on time and under budget.
About the Company
Originally formed as a limited-liability company in Georgia, Expression Therapeutics LLC began as owner of patents related to a gene therapy for hemophilia A and other platforms. To accommodate a new business model described above and to facilitate institutional capital investment, Mohan Rao became ET’s CEO.
First, a new privately owned holding company was incorporated in Delaware. Expression Therapeutics, Inc., has two wholly owned Delaware-registered subsidiaries: Expression Manufacturing LLC (EM, for manufacturing assets and personnel) and Expression Therapeutics LLC (for therapeutics assets and R&D). The latter focuses on driving scientific breakthroughs in expression-cassette optimization (ECO), which involves bespoke transgene bioengineering and protein engineering to enable development and optimization of gene therapy candidates for each target indication. EM manufactures viral vectors and cellular products for R&D and clinical use.
Scientific breakthroughs in transgene bioengineering and protein engineering enable ET to optimize a gene therapy for those target indications, initially focusing on bleeding disorders and oncology. Our clinical programs use both autologous and allogeneic cells as well as viral vectors. Our vector platforms include LV and adenoassociated viral (AAV) vectors. Combining the ECO platform approach with an understanding of target diseases, ET’s drug development platforms are unbeholden to any specific gene delivery technology. Based on proprietary genetic engineering of allogeneic healthy-donor γδ T cells, the company’s oncology platform alleviates many concerns associated with autologous αβ T-cell and gene therapies.
Timelines and Milestones
At the start of the EM project to build a new state-of-the-art manufacturing facility, no one could foresee the worldwide pandemic that would cause severe supply chain, workforce, and resource disruptions. Even with market uncertainties as COVID-19 was gaining momentum, the EM project’s success was ensured through commitment to collaboration and integrated project delivery with rigorous predesign and construction planning. That began with a clear understanding of the desired outcomes and constant participation throughout the process of major stakeholders (e.g., engineering and construction firms) along with the client company (ET):
• project management/design (CBRE Group of Chicago, IL)
• construction (Messer Construction of Dayton, OH)
• vendors (G-CON Manufacturing of College Station, TX, and others).
The greatest risks to the project schedule would come from massive global supply-chain bottlenecks and potential disruptions to personnel resources. Figure 1 shows the general project timeline, from the initial decision to build a new manufacturing facility to its completion. Major milestones appear in black, major scheduled events framing the project in blue, and major COVID-19 developments in red. Below we describe key activities in chronological order to show how the effects of COVID‑19 affected the overall timeline, discuss unique challenges experienced because of the outbreak, and highlight unique solutions or workarounds for remediating those challenges.
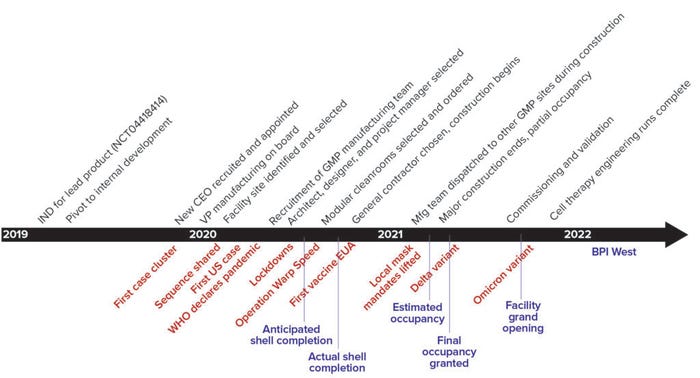
Figure 1: Timelines and milestones (major COVID-19 developments in red, scheduled milestones in blue, real timelines in black)
Site Selection
With its commitment to manufacture products internally rather than through a CDMO, ET’s pivot required finding an experienced expert to lead manufacturing operations, oversee facility construction, and build a CGMP manufacturing team. To fill that role, ET recruited William Swaney, who was director of a GMP vector production facility (VPF) in the translational core laboratories of Cincinnati Children’s Hospital Medical Center. Under his leadership, more than 70 lots of clinical grade viral vectors had been produced by the VPF and used in US and European clinical trials for clients ranging from institutional principal investigators to top-10 drug companies. With this leadership secured, one of the first orders of business was to determine a build site. Locations were considered in Chicago, IL; Atlanta, GA; and Cincinnati, OH. Each option had advantages and disadvantages, and after careful deliberation, management decided to locate the new facility in the Cincinnati area.
Home to nearly 12 million people, Ohio is the seventh largest economy in the United States and consistently ranks in the nation’s top-five business environments. Recognized as a “business-friendly” state, it recently has experienced steady growth in attracting biopharmaceutical companies. In 2019 and 2020, more than 40 projects involving expansion or build-out of bioscience companies had been announced. Some of that growth was attributed to prioritized governmental support available for bioscience companies.
Site selection occurred during the early spread of the COVID-19 pandemic but was not dramatically affected because travel restrictions, mask mandates, and lockdowns were not yet in effect. ET worked with two nationally recognized real-estate property management firms to find land available for purchase or lease based on a number of criteria: greenfield or building retrofit, proximity to similar business or technology parks, single or multistory design, and immediacy of availability. Because of the type of space needed, moving into an existing facility would have been overcomplicated.
An open warehouse space allowed the facility to be designed specifically for the needs of ET and EM. Additional site requirements associated with modular cleanroom implementation contributed to rapid phase-1 completion and optimal phase-2 design and future construction. Engagement of G-CON in prefabricating POD cleanroom suites before onsite construction was a strategic decision that helped maintain the critical path while reducing construction time and effort onsite. That allowed the contracting team to install required infrastructure to support the cleanrooms before the delivery date. G-CON worked with the Messer and CBRE teams to head off issues before implementing the POD cleanrooms and any problems that might arise while commissioning them during start-up.
A key construction concern turned out to be a shortage of material for fabricating circuit-breakers for main electrical panels and equipment. Messer circumvented that problem through alternative means and methods, including use of temporary power to continue start-up and commissioning for meeting the ambitious completion date. Messer also obtained temporary occupancy for the EM team so that support laboratories could be set up and training could be conducted to prevent delays in business operations, which otherwise could have extended the facility opening by at least four months.
Architecture and Design
The CBRE design team delivered a coordinated set of construction documents to meet the required basis of design (BoD). Pairing of accomplished design and project-management staff with subject matter expertise were drivers of design excellence for both the administrative and operations areas, as well as the heating, ventilation, and air-condition (HVAC) systems supporting the cleanrooms and laboratories. All design disciplines worked closely with Messer to resolve issues and help review alternatives. Construction was necessarily fluid and dynamic because of market circumstances. Design and construction teams worked together to find alternative solutions to meet the BoD and add value to the project. CBRE offered pragmatic and cost-effective solutions that otherwise might have been costly to implement during construction.
The design, construction, and ET teams collaborated to refine the schedule and budget without compromising the project’s intent. One prime example of a pragmatic solution came once the building permit was issued and the plumbing inspector requested reservoir sampling at the underside of sinks to monitor discharge. The teams brainstormed different solutions that ultimately kept the project on schedule and within budget.
Cleanroom Modules
EM reduced the overall timeline of facility start up with off-site–prefabricated modular cleanroom units. This allowed for robust facility selection and parallel-path construction for project success.
Design: The G-CON design process integrated an existing prefabricated unit into the EM facility layout and thus reduced the overall build time. The design consisted of three subPOD units (individual modules) that make up a process suite, a clean corridor, airlocks for materials and personnel, and locker rooms. The existing subPOD unit chosen for the process suite was housed at G-CON with a fully integrated building and environmental monitoring system. That was integrated with two additional subPOD units that were designed with a central control system for the entire combination. This design also accounted for future POD units to be added to the existing set using an integrated corridor.
Build: With the floor plan locked in at the time of purchase-order issuance, G-CON released material orders based on a standard submittal package provided and approved by EM to mitigate extended lead times during the pandemic. The robust POD design allowed for the transition of the HVAC system in an existing POD unit from a direct-expansion system with an electric reheat coil to a chilled-water and hot-water coil combination to align with the design intent of the host facility.
Test: G-CON POD units are subject to two testing processes that ensure system functionality and operational verification. G-CON uses an acceptance testing strategy to highlight the factory-acceptance testing (FAT) performed at its manufacturing facility before POD shipment along with final site-acceptance testing (SAT) verification once units are delivered, installed, and in the designed configuration within their host facility.
For EM, G-CON completed FAT, prepared POD units for shipment, and then stored them for five months until the host facility was ready for delivery. G-CON, EM, and CBRE coordinated to complete certain tests in person, thus allowing for verification of POD construction and utility connection locations. Because of the pandemic, G-CON accommodated virtual testing and deferred certain tests to the SAT stage based on a risks assessment of what would be verified during final testing.
Installation and Closeout: To mitigate against shut-down periods for onsite construction, POD units were integrated into the host facility building in just half a day through coordination among G-CON, CBRE, and Messer Construction. G-CON provided detailed documentation of requirements needed from the host facility to ensure that the units could be integrated for operation. G-CON, CBRE, EM, and Messer thus were able to mitigate potential delays to site install by scheduling POD delivery around facility power availability. Messer provided G-CON with temporary power to enable unit installation and preliminary testing.
Once the cleanrooms were installed, G-CON automation and commissioning worked with the host facility’s trade partners to ensure integration of the onboard automation system with that of the host facility. A data-logging scheme enabled storage and sharing of operational critical information with the EM cloud server for continuity.
Groundbreaking and Construction
Execution and administration of the overall construction project were carried out with both thoroughness and expediency. Weekly owner–architect–contractor (OAC) meetings helped prioritize tasks to keep the project on time, identify foreseeable issues (such as pandemic supply-chain disruptions), and make clear action plans to mitigate each problem. Based on best practices for construction administration, CBRE and Messer worked diligently together to prioritize submittals and requests for information and stem delays of products and installation sequence. Messer prioritized scheduling of construction activities, submittals (long lead items), and sequenced tasks to prevent wastes of time in waiting, double-handling of materials/equipment, and storage. The construction firm facilitated receipt of deliveries and tracking for EM using an open area, enabling resources to be deployed on time when needed.
Construction was executed with high quality in mind. Messer and CBRE used quality assurance and control (QA/QC) to validate work in place and meet specified standards. Messer provided timely corrections to all deficiencies upon discovery, thus preventing the need for a long punch list. CBRE and Messer worked out such issues together and in person during site visits, thus keeping the schedule on track.
During preconstruction and before finalizing the contract with EM, Messer and the design team worked collaboratively to get submission and approval for long lead items related to a backup generator, air-handling units, dedicated outside air system (DOAS) units, electrical gear, and switch panels. Doing so enabled Messer to order long-lead equipment immediately upon contract approval. That arrangement presaged some impacts that the pandemic would have on supply chains and ultimately proved to be a crucial component of completing this build-out on time.
Planning ahead and working together would be important throughout construction. To address market fluctuations in cost and availability of some construction materials, Messer used the facility’s 40,000-ft2 open warehouse to preorder and store many materials needed for the overall project. Historically, such materials would have been ordered as needed and just in time.
Problems and Solutions: All structural metal studs were ordered at the very beginning of the job. Before the COVID-19 outbreak, such materials were readily available with typical maximum lead times of one or two weeks and at relatively static costs. During the height of the pandemic, lead times expanded to six-to-eight weeks, and costs increased monthly. Similarly, all drywall for this job was ordered early on to take advantage of bulk pricing and “lock in” the price at a lower rate. In addition to pandemic disruptions, availability of drywall supplies also had been impacted by the freezing weather of February 2021 in Texas, which severely exacerbated the dearth of such finishing materials. Flooring and specialty items such as doors and hardware also were procured early and stored until needed for installation.
In a build-out like this one, most equipment that eventually would be placed into laboratories and cleanrooms (e.g., incubators, freezers, refrigerators, and biosafety cabinets) normally would be ordered based on established lead times for delivery after major construction has finished. In this case, however, most equipment was procured early on to minimize delays, and as construction proceeded, equipment was received and stored in the warehouse to prevent delays.

Pandemic Challenges
In many ways, COVID-19 has turned the world upside down. The pandemic caused a workforce shortage across all industries, including the factories and warehouses where products/materials are manufactured and assembled. That grossly inflated lead times and material costs. For construction items such as metal studs, lumber, drywall, doors and frames, garage doors, electrical components, and HVAC equipment — all readily available in the past — lead times increased by weeks and even months. Some manufacturers could not even give an expected lead time for certain materials/equipment.
In addition, unprecedented monthly price increases were passed down from material manufacturers and suppliers to contractors, with little ability to lock in pricing. Focusing on and ordering long-lead equipment as soon as possible ensured that it would be ready on time without accommodating costly price increases. Messer used the facility’s large, open warehouse space to order and store as many materials as possible to ensure that they would be on site when needed.
Despite measures to anticipate challenges that the pandemic would present, this project was not immune to challenges caused by material availability. Because of missing circuit breakers, for example, Messer and the construction team were forced to put in place temporary power solutions to perform SAT for the G-CON POD units. The team also implemented measures for a temporary certificate of occupancy when aluminum storefront doors, garage doors, and circuit breakers were not shipped on time. That enabled ET to open its doors for operation as planned (Figure 1).
Successful COVID-19 Plan at Worksite: The ability to staff a construction project properly during the pandemic was a major challenge. Messer and its subcontractors were able to staff the job adequately with teams that could act quickly and decisively when issues came up on site.
Once workers were present, the team put protocols in place to keep them safe as the job moved forward. Those included daily COVID-19 screening, social distancing, washing and cleaning of shared hand tools, masks and personal protective equipment (PPE), and scheduling practices to prevent “stacking” of trades working in the same areas at the same times. When most of the world either shut down or evolved into a “work-from-home” scenario during the height of the pandemic, the construction team remained steadfast and came to work safely every day. Messer Construction (Columbus, OH), Kings Electric (Lebanon, OH), TJ Dyer (Cincinnati, OH), and Cinfab (Cincinnati, OH) had 40–50 workers on site every day working together on this project with no lost time or incidents of COVID-19.
Virtual Meetings: The pandemic has forced people of different generations to work remotely using Zoom, Bluebeam Revu, screen sharing, and so on. With technical support, EM, Messer, and CBRE managed open communications with the real-estate developer (NorthPoint Development) through design meetings including participants in New York, Chicago, St. Louis, Atlanta, Pittsburgh, and Cincinnati.
Managing Process Development
One product candidate identified for clinical development at the founding of EM was an allogeneic cell therapy for neuroblastoma. Teams at Emory University and Children’s Healthcare of Atlanta recently had completed a preliminary IND meeting with the US Food and Drug Administration (FDA). Based on positive feedback from that interaction, the project was fast-tracked for completion of clinical trial design, preclinical pharmacology/toxicology studies, and clinical materials manufacturing. ET was eager to conduct process development work on that and other high-priority candidates.
The pandemic was changing such work throughout the industry as many raw materials commonly used were prioritized to Operation Warp Speed development programs. Travel disruptions (especially by air) were numerous, and many staff members were reluctant to travel, lodge at hotels, and work at other locations with the associated risks of infection. The company was faced with a challenge of securing laboratory space to conduct needed translational and process-development experiments as facility construction progressed. Along with the strategic vision to build in-house manufacturing capabilities, ET had undergone a major increase in overall staffing. With about two-thirds of the new personnel engaged in research and proof-of-concept studies, it soon became apparent that the GMP manufacturing team would need to work closely with the R&D team to translate its methods into an acceptably CGMP-compliant process while developing and qualifying the necessary analytical assays needed to support product manufacture. With existing facilities in Atlanta, GA, the first obvious solution was to have the GMP team travel between there and Ohio to perform such studies. EM’s central location allowed for travel flexibility by air or land based on staff preferences during the pandemic.
Several runs were performed successfully using that strategy, but it proved to be nonideal for the circumstances. The primary considerations for that determination were related to the constantly changing circumstances of the pandemic — e.g., waves of high infection incidence and guidance provided by local and national organizations regarding COVID-19 safety. Travel between Ohio and Georgia also limited the amount of time that people could dedicate to nonexperimental activities such as standard operating procedure (SOP) writing and protocol development.
Unfortunately, the Cincinnati region lacked adequate wet-laboratory incubator space for start-ups and small biotechnology companies. Given the urgent need to perform development work and support proper work–life balance for staff during the pandemic, EM pursued a unique strategy. Many local institutions of higher learning were closed by the pandemic or engaged in remote learning only, so we reasoned that some of those institutions could be open to renting out laboratory space. Almost all of the several universities we contacted responded, generally one of four ways:
• no interest in pursuing
• interest but unsure how long facilities would be available or how to manage such a contract
• interested but with concerns about the work being proposed (e.g., indemnification)
• interested and open to further dialogue.
We focused on the latter, of course, and one university agreed on a reasonable charge to rent to EM a medium-sized biosafety-level 2 (BSL-2) laboratory with incubators, biosafety cabinets, refrigerators, freezers, an autoclave, and storage space. Contract negotiations took just over a month to complete. EM personnel had to complete institutional training and submit an institutional biosafety committee (IBC) protocol for the proposed scope of work. They also were responsible for cleaning, calibration, and maintenance of equipment as well as stocking the laboratory with materials and small instruments such as microscopes, water baths, welders, sealers, and so on. We occupied the laboratory for just over six months.
Work was performed at the rented laboratory up to and during the initial build-out and beginning of operations at the new facility after major construction had been completed. This creative solution was cost effective, well received by staff, responsive to pandemic concerns (requiring mask wearing, daily screening, and social distancing), and helpful in limiting the number of personnel on site at the new facility. In addition to enabling crucial work to be performed in parallel as construction progressed, the exercise also introduced EM personnel to university staff who were in positions to assist with the future development of internship programs. That link enabled the university administration to introduce newly graduating students who were entering the job market to Expression Therapeutics.
Office Solutions: During construction of the new facility in the height of the pandemic, EM still needed to perform process development work (as described above) and to create, review, approve, and issue all controlled documents required (e.g., SOPs, protocols, and raw material specifications). We needed to ensure that we could hit the ground running when our facility was complete and we could take occupancy. After considering the lease of class A office space for that purpose, which would have been short term amid ongoing pandemic concerns, staff members chose to use Microsoft Teams as a “digital office.” It allowed all staff to be present in a virtual office, working independently but simultaneously during normal business hours. Not only could we communicate through the chat function, but we also opened meetings and created breakout rooms so staff members could meet all together or in smaller groups to discuss document assignments and provide real-time reviews to offer feedback for improvement. This unique solution minimized delays in document creation by putting all key contributors and approvers in the same virtual space, where nearly instantaneous revisions and corrections could be made — rather than scheduling meetings or sending multiple emails and waiting for responses.
As construction neared completion, Messer allowed EM to share space in its jobsite trailer, which provided an adjacent office area just outside the facility. This arrangement provided a space to conduct on-site staff meetings, work face-to-face on documents as mentioned above, and meet with suppliers and vendors of products or materials for the facility. It also enabled personnel to tour the accessible parts of the facility. Once EM was granted partial occupancy of the space, daily operations were moved from the trailer and digital office to the completed office area. Visitors and personnel working on site followed all COVID-19 restrictions and procedures that had been in place at the start of construction.
Quality Management
When EM first began planning its new facility, we decided to implement a modern, paperless quality management system. Our major investment in the Ohio operation represented a new phase of growth, and we saw document-control efficiency as a key challenge. EM needed a system that would reduce the amount of time needed to move each document from creation through review, approval, and issuance. We met with multiple electronic quality management system (eQMS) vendors to evaluate their capabilities, pricing, and customer support — ultimately choosing Qualio (San Francisco, CA) over other platforms for its combination of powerful functionality and usability.
Visibility into metrics is a key requirement for data-driven organizations. Previously, ET had tracked deviations, corrective and preventive actions (CAPAs), and training in spreadsheets and hard-copy documents. With Qualio software, the company could unite all data in one place and easily find areas to improve.
EM was impressed with the program’s ease of use from validation to implementation. The EM team was excited by how easy it was to use in creating documents, templates, and events, and in following deviations and CAPAs to closure. Qualio helped EM hit the ground running with the opening of this new facility by helping personnel maintain documents during remote work. With the use of this cloud-based eQMS during the pandemic, productivity was unaffected because staff could write, review, approve, and issue documents remotely. Thanks to the digital repository and regulatory-compliant electronic signatures, the quality team no longer had to spend time on laborious document management.
Knowing that the transition from paper to electronic documents could be difficult, EM relied on the Qualio Plus team to help with the digitization and implementation of document templates for eQMS use. Whether EM had questions about transitioning from paper or about audit readiness, Qualio Plus provided ongoing support. For example, when EM needed help creating events to track items outside of deviations and CAPAs — e.g., cleaning schedules and procedures — support from Qualio experts made it work. During the evaluation phase, EM could see the value of Qualio’s commitment to quality and support. That has carried through now that we’re an established customer. Some other vendors made users feel like just “number,” but this is more like a true partnership and has changed our work for the better.
Engineering Runs
On 25 October 2021, the project management group provided a certificate of substantial completion to the contractor. That date coincided with issuance of a full certificate of occupancy from the county. These accomplishments paved the way for EM personnel to initiate operations within the new facility. Because of previous efforts to mediate pandemic disruptions during build-out, personnel management, and developmental work (along with having written most necessary controlled documents for operations with approval in the eQMS), the team now could focus on setting up cleanrooms and laboratories, then commissioning and validating needed systems. Some systems or pieces of equipment were validated in house; other validations were outsourced and executed by system manufacturers or their subsidiaries. Process and assay-development work that had been performed at ET’s Atlanta site or locally at the rented laboratory space accelerated completion of remaining experiments before execution of our engineering runs at scale.
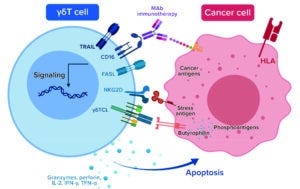
Figure 2a: γδT-cell immunotherapy mechanisms of action to enhance neuroblastoma tumor regression
CD16 = FcγRIII, a cluster of differentiation (CD) protein found on the surface of natural killer cells, neutrophils, monocytes, macrophages, and certain T cells
FasL = a type-II transmembrane protein that belongs to the tumor necrosis factor (TNF) family, also known as CD95L or CD178
HLA = human leukocyte antigen
MAb = monoclonal antibody
NKG2D = an activating receptor that is mostly expressed on cells of the cytotoxic arm of the immune system
γδTCL = gamma-delta T-cell lymphoma
TRAIL = TNF-related apoptosis-inducing ligand (TRAIL), a cytokine secreted by most normal tissue cells that functions as a ligand to induce apoptosi
The company was now in position to complete development and in-house manufacturing of an allogenic γδT-cell–based therapeutic (Allo-γδ-T cells) against recurrent/refractory NB (Figure 2a). Allo-γδ-T cells alleviate concerns of long-term cell ablation and tonic signaling while presenting little risk of graft-versus-host disease (GVHD) and offering the potential for safe, repeated dosing with enhanced cytotoxic activity through synergy with existing therapies.
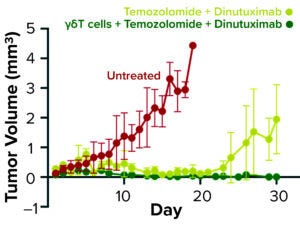
Figure 2b: Tumor volume in a neuroblastoma mouse model over time stratified by treatment regimen
As Figure 2b shows, untreated mice in a mouse NB tumor model showed tumor progression and died by 20 weeks. Treatment of NB mice with standard-of-care agents extended their survival but failed to prevent tumor growth completely. However, when γδ T cells were added to the standard chemotherapy–immunotherapy regimen, survival was extended further through complete tumor eradication. One FDA IND requirement for this product was that the manufacturing facility complete at least three full-scale engineering runs to establish the robustness of its proposed manufacturing platform.
Apheresis products from three prescreened healthy donors were used as the starting materials for each of those runs. Through a relationship with a large international blood-collection agency, ET continuously screens blood-cell samples from healthy donors to identify those whose cells can be expanded favorably using our process. Those donors can be recalled for full-leukopak donations to provide starting material for the cell-therapy product pipeline.
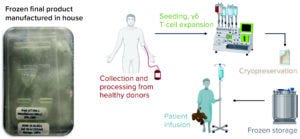
Figure 3: Cell therapy engineering run overview; overview of cell manufacturing process start to finish
Briefly, as Figure 3 shows, blood mononuclear cells are isolated from leukapheresis material and cultured under proprietary conditions in media free of serum and animal-derived components, depleted of αβ T cells, formulated in cryopreservation media, filled into bags using a closed system, and frozen in a controlled-rate freezer (CRF). Using a developed freezing protocol enables preservation of cellular integrity during the cooling process to reach the cryopreservation temperature. Frozen cells are transferred and stored in vapor-phase liquid nitrogen under GMP conditions. Release testing of the final cell products from all three engineering runs was performed in our newly established QC laboratory. Critical quality attributes (CQAs) were measured across the three engineering runs, and the cell products were tested in requested toxicology and distribution studies for IND submission. Thus, EM had achieved the successful GMP-stage manufacturing quality and consistency expected of a state-of-the-art manufacturing facility for cell and gene therapies.
Success Against the Odds
The COVID-19 pandemic was a disruptive event that spread quickly around the globe and fundamentally altered not only the biopharmaceutical industry, but also the rest of the world. The requirements and resources needed to build, equip, qualify, staff, and operate a modern cell and gene manufacturing facility are challenging even under ideal circumstances. The pandemic situation was extremely daunting in the form of massive global supply chain challenges and human resource shortages that could have derailed our entire project. This situation necessitated a paradigm shift in how to approach management of personnel, resources, time, and supply chains for construction materials, equipment, raw materials, and process development. With hard work, a good team, and flexibility in our approach, we were able to complete the build-out of our facility without injury, on time, and under budget — and initiate manufacturing operations in the new manufacturing facility shortly after opening. These results are noteworthy especially because neither EM nor any of the engaged team of engineers, contractors, and personnel experienced COVID-19 infection or lost any days to pandemic outbreaks.
Carl Graves ([email protected]) is senior project manager in LEED AP, and John Nguyen ([email protected]) is associate director for life sciences midwest at CBRE in Chicago, IL. Corresponding author William Swaney ([email protected]) is president, and Eric Day ([email protected]) is director of quality assurance at Expression Manufacturing LLC, 4692 Brate Drive, West Chester, OH 45011. Kim Muro ([email protected]) is director of business development, Idrees Hamed ([email protected]) is director of project management, and Tim Rasmussen ([email protected]) is a design consultant for G-CON Manufacturing, Inc. in College Station, TX. Eric Davis ([email protected]) is a project executive at Messer Construction Company in Dayton, OH.
You May Also Like





