G-Protein–Coupled Receptors: Promising Targets for Antibody–Drug Conjugates
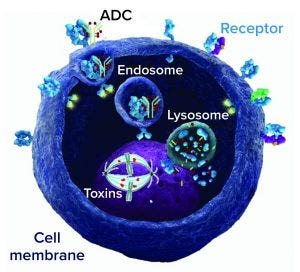
Antibody–drug conjugate (ADC) mechanism of action; an ADC binds to a cell-surface target receptor, which is internalized to form an endosome and merge with a cell lysosome. Drug is released from the lysosome and acts on its target (DNA or microtubule) to kill the cell.
G-protein–coupled receptors (GPCRs) are a large and diverse family of seven-transmembrane–domain proteins expressed on the surface of human cells. These molecules respond to external stimuli by initiating signal-transduction pathways that affect the expression of a large family of genes — which, in turn, regulate a range of vital physiological processes and functions. Figure 1 illustrates the general pathways of GPCRs.
Without these proteins, humans simply could not survive: Without β-adrenergic receptors, we could not regulate our blood sugar, for example; and without serotonin receptors, we could not experience happiness. Many physiological functions are related to the signaling pathways regulated by GPCRs, and dysfunctions of those pathways cause a number of diseases such as cancers, mental disorders, diabetes, cardiovascular diseases, and inflammatory disorders. Therefore, GPCR proteins are a “hot topic” of research, both in basic scientific research and as drug targets in the pharmaceutical industry.
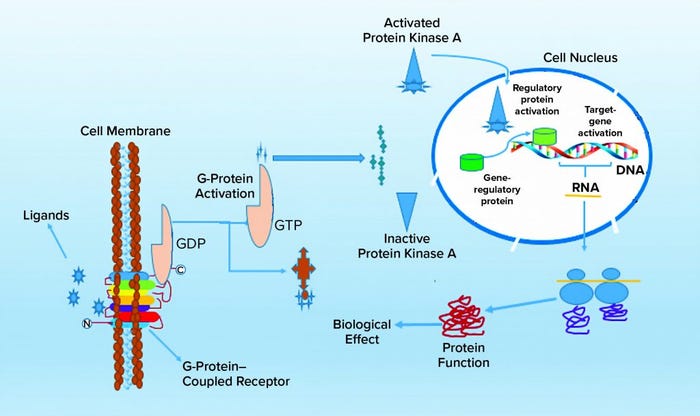
Figure 1: Molecular signaling pathways of G-protein–coupled receptors (GCPRs)
“If you have GPCR[s], you have the world,” says Hualiang Jiang, director of the Shanghai Institute of Materia Medica, part of the Chinese Academy of Sciences. More than 800 members of the GPCR protein family have been identified so far, and sales of drugs targeting GPCRs account for 27% of the global drug market. The “Example Drugs” box below lists some GPCR-related drugs that already are commercialized, some as prescription medications and others sold over the counter. They have saved lives, reduced pain, and restored healthy function to many patients.
Example Drugs Targeting GPCRs |
|---|
Cardiovascular: atenolol, carvedilol, clopidogrel, enalapril, losartan, metoprolol, terazosin, valsartan Psychological: bupropion, buspirone, fluoxetine, olanzapine, paroxetine, risperidone, sodium valproate, venlafaxine Gastric: famotidine, ranitidilne Hormonal: goserelin, leuprorelin Respiratory: albuterol, ipratropium bromide, montelukast, salmeterol Allergy: cetirizine, fexofenadine, loratadine Others: latanoprost, propofol, sumatriptan |
Using X-ray crystal diffraction technology, scientists have elucidated the atomic structure of many GPCRs, such as β2-adrenergic receptors related to cardiovascular diseases and diabetes, adenosine A2A receptors for cancer immunotherapy, the CCR5 chemokine receptor for immune-system regulation, the P2Y1R purinergic receptor related to thrombotic disease, and the D3 dopamine receptor that regulates behavior and mood. All those are examples of important therapeutic targets for treating human disease. Their structural information provides a theoretical basis for subsequent drug development.
Data from as early as 2017 showed that the US Food and Drug Administration (FDA) had approved a total of 475 GPCR-targeted drugs, accounting for 34% of all US-approved pharmaceuticals (1). Even with so many such drugs already on the market, pharmaceutical research toward developing new drugs targeting more members of this large protein family is growing ever more intense. Indications for targeted GPCR drugs also are expanding from traditional indications such as hypertension, allergies, anesthesia, and schizophrenia to new areas such as Alzheimer’s disease and obesity. Currently 321 targeted GPCRs are in clinical trials, of which 66 (20%) drugs target novel GPCR targets.
In addition to the new GPCR targets, new approaches to targets both old and new are being explored. So far, most drugs developed to target GPCR molecules are small molecules and low–molecular-weight peptides. Monoclonal antibody (MAb) drugs, however, offer many advantages over small molecules and peptides, particularly for oncology and immunology indications. Advantages for targeting GPCRs include
target specificity and improved safety profiles (immunogenicity can be a safety concern, but human or humanized MAbs can reduce that greatly)
long in vivo half lives
ability to eliminate target cells through effector functions such as antibody-dependent, cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP).
Several different types of antibodies can target GPCR proteins: e.g., single-domain antibodies, bispecific and multispecific antibodies, and antibody–drug conjugates (ADCs). So the pharmaceutical industry’s interest is increasing for development of MAb drugs targeting GPCR molecules for treating GPCR-related diseases. At least 74 scientific research projects are focusing on 37 GPCR molecules. A total of 57 MAbs are in discovery and preclinical research stages, and 39 have entered clinical studies. Table 2 lists some of them. Three such MAbs have been approved: galcanezumab (Eli Lilly), eptinezumab (Lundbeck), and fremanezumab (Teva), all of which target the GCPR ligand calcitonin gene-related peptide (CGRP) instead of the receptor itself. A fourth approved drug — erenumab (Amgen and Novartis) — targets the CGRP receptor (CGRPR).
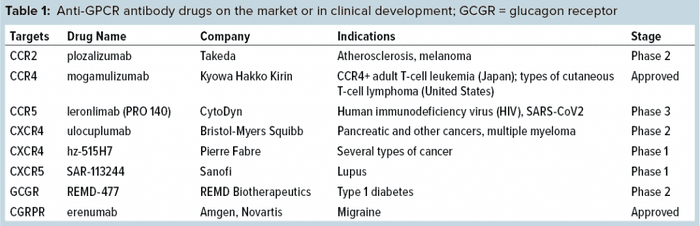
As Table 1 shows, antibodies against GPCRs achieve their therapeutic function through two main mechanisms of action (MoAs). One is to block GPCRs from binding to their ligands, thus preventing the signal transduction causing disease. Erenumab is a good example. CGRPs and CGRPRs both play integral roles in the pathophysiology of migraine.
The other MoA is antibody-mediated killing of cells expressing a given receptor through so-called effector function. For example, mogamulizumab (Kyowa Hakko Kirin) is specifically engineered to exhibit enhanced ADCC activity toward CCR4-expressing cancer cells. Because GPCR expression is widespread, and because the proteins mediate a number of functions, antibodies to GPCRs often have been explored for multiple and even seemingly unrelated indications. For example, CytoDyn recently announced that its human immunodeficiency virus (HIV) entry inhibitor leronlimab targeting CCR5 also shows promise for mitigating the cytokine storm of COVID-19, for which it will be tested in phase 2b–3 trials.
Although through effector functions a MAb can kill cancer cells that overexpress the antigen that it binds to, such killing can be enhanced further by attaching to a traditional antibody drug a highly potent toxin, creating an antibody–drug conjugate (ADC). Once an ADC binds to a target tumor cell, it will be internalized, where the conjugated potent chemical toxins can be released to kill the cell. Figure 1 illustrates this MoA. Meanwhile, normal cells expressing little to no such antigens are spared. So ADCs increasingly have been favored by researchers, setting off a wave of research and development worldwide.
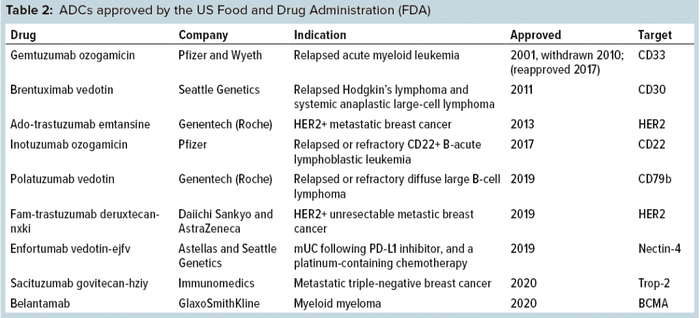
Development of ADCs has been a long and hard journey spanning well over 20 years now, with a number of successes and failures along the way and much learned through technical innovations and advancements. Because an ADC is made up of three parts — antibody, toxin, and a linker molecule that connects the two — it is more complex to develop, manufacture, and test than regular antibodies and other recombinant proteins. Nevertheless, eight ADCs have been approved by the FDA (Table 2), half of those within the past year. ADC research and development are active even beyond the oncology space, where all of the current eight approvals have resided so far.
GPCRs as ADC Drug Candidates
Even though GPCRs represent the largest family of protein targets for approved drugs, they have not been exploited much as therapeutic targets for cancer, with the exception of certain endocrine and hormone-responsive tumors. Meanwhile, even though tremendous progress has been made, the need to develop new therapies for cancer treatment is ever increasing. With improved understanding of the expression, structure, and functions of GPCRs in cancer, new therapies targeting them for cancer treatment will emerge.
GPCRs with increased expression in cancer cells should be particularly attractive as ADC targets. In fact, upon binding to a ligand or an agonist antibody, most GPCRs will initiate a conformational change that leads to internalization into the cell. The rapid internalizing of GPCR is a key feature to turn it into an effective ADC drug target. A fluorescence automated cell sorting (FACS) screen can help scientists discover antibodies with such an agonist profile for potential as ADC candidates. Furthermore, certain tumor cells highly express mutated GPCRs, such as those lacking one or two transmembrane domains. If antibodies specific to such mutated GPCRs could be isolated, they might be developed into effective ADC drug candidates that would spare normal cells expressing the normal counterpart protein.
LGR5 is the first GPCR family member to be targeted by an ADC that demonstrated potent tumor efficacy and safety in vivo (2). Furthermore, this ADC decreased tumor size and proliferation, providing improved survival in a genetically engineered model of intestinal tumorigenesis. The developer hopes that clinical trials can demonstrate its promise further in the near future.
DS-6157 is a “first-in-class” ADC drug targeting GPR20, an orphan GPCR that is expressed specifically in gastrointestinal stromal tumors (GIST). DS-6157 has a different MoA from that of TKI and can be used to treat GIST patients who develop resistance to TKI treatment. DS-6157 currently is in phase 1 clinical trials.
LegoChem Biosciences (LCB) has chosen three strategic GPCRs to use as drug targets. Based on a site-specific and plasma-stable ADC platform, LCB’s technology uses a short extra amino-acid (CAAX) sequence that creates a site-specific conjugation site at the termini of antibody chains. A prenyltransferase attaches the isoprenyl substrate to the cysteine residue of the CAAX sequence, generating a conjugation site for linker attachment. Through CAAX-mediated prenylation, both the site of drug conjugation and the number of drugs conjugated to the antibody can be controlled precisely, enabling developers to obtain ADC molecules with a specifically defined drug–antibody ratio (DAR). Sorrento Therapeutics has developed three GPCRs using this novel ADC format to target CCR2, CXCR3, and CXCR5. Based on published data, those ADCs show that the potency of IC50 in the ranges of 1.0 nM (3).
Development of GPCRs as ADC targets will face several challenges arising from the unique properties of GPCRs as seven-transmembrane glycoproteins in addition to the overall challenges of developing ADCs as therapeutics. It is simply more difficult to obtain specific and high-affinity antibodies to GPCRs because they are not soluble under ordinary conditions, with only limited extracellular epitopes assessible to antibodies. However, some companies are developing innovative strategies to screen for antibodies that will be specific for GPCRs. Newer ADC technology platforms and successful clinical experience in ADC development will those companies overcome the related challenges. As Table 2 shows, a number of companies are in the process of developing ADC formats as improved drug candidates for cancer treatment. MAbs with agonist properties should be more effective than other antibodies for use in the ADC format. We hope to see more ADCs targeting GPCRs for treating cancer and other life-threatening diseases in the future.
References
1 Hauser AS, et al. Trends in GPCR Drug Discovery: New Agents, Targets, and Indications. Nat. Rev. Drug Discov. 16(12) 2017: 829–842. https://doi.org/10.1038/nrd.2017.178.
2 Junttila MR, et al. Targeting LGR5+ Cells with an Antibody-Drug Conjugate for the Treatment of Colon Cancer. Sci. Translat. Med. 7(314) 2015: 314; https://doi.org/10.1126/scitranslmed.aac7433.
3 Safe Harbor Statement. Sorrento Therapeutics October 2013; https://www.sec.gov/Archives/edgar/data/850261/000119312513390756/d607708dex991.htm.
Further Reading
Hutchings CJ, Koglin M, Marshall FH. Therapeutic Antibodies Directed at G Protein-Coupled Receptors. mAbs 2(6) 2010: 594–606; https://doi.org/10.4161/mabs.2.6.13420.
Gingrich J. How the Next Generation Antibody–Drug Conjugates Expands Beyond Cytotoxic Payloads for Cancer Therapy. ADC Review 7 April 2020.
Kumar KK, Burgess AW, Gulbis JM. Structure and Function of LGR5: An Enigmatic G-Protein Coupled Receptor Marking Stem Cells. Protein Sci. 23(5) 2014: 551–565; https://doi.org/10.1002/pro.2446.
Corresponding author Andrew C. Huang, PhD, is chief scientific officer ([email protected]); Deling Yu is a research associate in the processes department; Yuqing Shen is senior business development manager; and as of this writing, Yongjian Wu, PhD, was chief operating officer of MabPlex USA, 10210 Campus Point Drive, San Diego, CA 92121.
You May Also Like





