Optimization for Biopharmaceutical Manufacturing
July 1, 2008

innovatis has established a technology portfolio to assist with the implementation of process analytical technology (PAT) at pharmaceutical companies focused on manufacturing biologics. PAT is a critical component in FDA’s initiative entitled, “Pharmaceutical CGMPs for the 21st Century: A Risk-Based approach,” which was announced in August 2002. This initiative is intended to support innovation and efficiency in pharmaceutical development, manufacturing and quality assurance. PAT is a system of building into processes “quality by design” (QbD), an idea that product quality begins with a deep and sophisticated understanding of the scientific and engineering principles involved in a manufacturing process and the identification of variables that affect quality. Product quality cannot be “tested into” a product; it must be built in from the beginning.
Quality by design, according to innovatis: ()
Our goal is consistent with QbD and PAT: innovatis wants to help modernize the way laboratories use and manage their sample monitoring data. Because monitoring data are recorded more frequently in process designs that follow QbD and PAT guidelines, such processes can be understood with better precision, which enables quicker and easier adjustments. This precision helps optimize communication between authorities and industry, minimize compliance and validation efforts, maximize production, and ultimately bring new drugs to market faster.
The Ultimate Goal
The goal of “real-time release” is to increase a company’s confidence in the quality of each batch produced and delivered — and to assure faster product clearance. To achieve these goals, time devoted to sampling tasks and QC reviewing must be reduced. For certain applications, sensor-based measurements provide a useful process signature that may be related to underlying process steps or transformations. Based on the level of process understanding, these signatures may also be useful for process monitoring, control, and end-point determination when they relate to product and process quality.
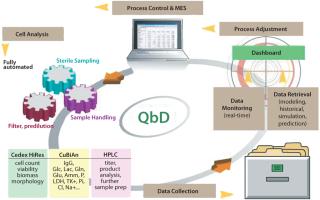
Cell counters, for instance, have been considered “off-line” devices without the capability of real-time process monitoring and control. innovatis’ goal is to change that. Networked analytical instruments such as cell counters, biochemistry analyzers, HPLC systems, and other devices will play an important role in biopharmaceutical processes as data transfer becomes paramount for quicker and safer product clearance.
An Elegant Solution
In cell culture production, a deep understanding of the fermentation process is necessary to maximize yields. Companies need to understand the critical parameters that substantially influence fermentation and the effect changes to these parameters have on their overall processes. In addition to physical parameters such as temperature and pressure, it is vital to monitor the change over time in cell count, viability, and substance concentrations. Continuous, round-the-clock monitoring of these variables is necessary to enable targeted control of fermentation processes. Temperature, pressure, and pH may be directly measured. Measurement of other, more complex fermentation parameters requires sterile extraction and handling of samples and preparation steps including filtration and predilutions.
The Holistic Approach
innovatis has established a team of experts to bring together the hardware, software, and intellectual property necessary to deploy fully automated cell culture analysis in a process environment. Our approach involves holistic solutions consisting of laboratory instruments, software tools, and interfaces to existing customer systems. innovatis has gained a broad understanding of the strict requirements for regulated environments from years of working with customers in pharmaceutical production. We offer a complete service package for those who wish to integrate PAT in a validated environment.
Sterility requirements for fermentation processes are extremely stringent. This is particularly true for sample extraction because there is a risk of contamination each time a sample is taken. BaychroMAT® CellCount from Bayer Technology Services is a fully automated system capable of sterile sample extraction directly from a bioreactor. Developed specifically for fermentation processes, it performs cyclical extraction and automated analysis of defined, representative samples of the bioreactor contents with no risk of contamination.
As an automation platform, BaychroMAT supports seamless integration of the Cedex™ or Cedex HiRes cell count analyzers from innovatis to enable measurement of cell count and viability. Cedex automated cell analyzers employ the Trypan Blue exclusion method for determining cell concentration and viability within cell cultivation and fermentation processes. Cedex HiRes works with the digital images of cells to make available morphological parameters such as diameter and compactness, as well as the amount of aggregation found in cell samples.
In addition to cell count and viability, IgG production and metabolite levels are important information for monitoring process control. CuBiAn, also from innovatis, is a photometric biochemistry analyzer that can link with the BaychroMAT CellCount platform. CuBiAn provides robust, high-throughput analysis based on a membrane-free operating principle. All data are easily transferable to LIMS for adherence to established QbD requirements.
Open System Management
The open system concept innovatis uses accommodates integration of additional analytical instrumentation (e.g., protein assays and chromatography systems) to the platform. The architecture is expandable to add monitoring capabilities, as well as compatible with existing data output.
Control of the automated measurement process is enabled by ARTS-SC software, which manages interaction of all system components. The platform can be connected to bus protocols and all common process control systems (analog and digital) through ARTS, the integrated Analyzer Result Transfer Software. The system also includes a self-monitoring function with generation of the corresponding status signals. All measured data are thus directly usable for automated process management.
With the combination of software and the sterile sampling of the BaychroMAT® platform, it is now possible for the first time to consistently control fermentation processes automatically by online measurement of cell count, viability, and other complex parameters provided by CuBiAn. The availability of an extensive system suitability test concept assures reliability of measurement data and therefore accurate real-time monitoring and modeling. All parties including biopharmaceutical manufacturers, regulatory authorities, and international societies agree that “device-to-device” accuracy is a key to achieving their objectives in the real-time release approach.
You May Also Like





