Collaboration Will Drive Regenerative Medicine: Toronto Development Center Will Help to Advance the Field
April 18, 2016

Xuri cell culture media are formulated for the growth and expansion of human mesenchymal stem cells after their isolation from a number of sources.
With support from the federal government of Canada, GE Healthcare and the Centre for Commercialization of Regenerative Medicine (CCRM) are pushing into new frontiers to advance the progress of cell therapy and regenerative medicine. When I first met Michael May, president and chief executive officer (CEO) of CCRM, both our organizations had been exploring opportunities in parallel to drive the cell therapy industry forward. CCRM’s mission is to create and sustain a global nexus for company creation, technology and cell therapy development, and clinical trials in regenerative medicine. The goal of GE’s cell therapy business was to introduce solutions and tools to the cell therapy industry and thus enable scalable, commercialized manufacturing of such therapies. Combining our common interests and complementary expertise seemed opportune.
With a $40 million (Canadian dollars) investment from GE and the government of Ontario, CCRM and GE are building a center for advanced therapeutic cell technologies. It will be a place where therapy manufacturers and developers can solve problems together with experts in the industry and come up with crucial manufacturing technologies and workflow solutions that could enable industrial-scale cell therapy manufacturing. The creation of this center, which we named BridGE@CCRM, marks a milestone in the progression of the cell therapy industry. Here’s why it matters.
Just What the Doctor Ordered
Modern medicine has advanced dramatically over the past few decades. With the invention, development, and commercialization of biologics, we moved from a blanket approach (treating many diseases using small molecules) to the use of more specific biologics with fewer off-target effects. Today, we could be facing another paradigm shift with the use of injectable engineered cells as therapies: treatments that promise transformative, durable responses or even cures.
Research, early stage testing, and clinical trials are showing extremely positive results for this new kind of medicine. A major focus of current attention is chimeric-antigen receptor (CAR) T cell immunotherapies. Clinical trials involving CAR-T cells have offered cancer patients who have exhausted traditional chemotherapy treatment options a new lease on life. Some patients have come through early stage trials to be declared effectively cancer free. In a recent study on T-cell therapy for blood cancers conducted by the Fred Hutchinson Cancer Research Center in Seattle, WA, 94% of participants with acute lymphoblastic leukemia (ALL) saw their symptoms vanish completely. Patients with other blood cancers had response rates >80%, and more than half of study participants experienced complete remission (1).
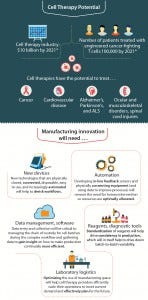
Figure 1: The future and promise of the cell therapy industry
Growth projections and investor activities tell a similar story about the regenerative medicine industry’s promise (Figure 1). The global market for cell-based therapies is expected to surpass the US$20 billion mark by 2025, with an annual growth rate of 21% (2). More than 631 cell therapy clinical trials are currently active, targeting indications such as cancer, heart disease, diabetes, chronic wounds, neurodegenerative diseases, stroke, spinal cord injury, vision impairment, and severe burns (3). Biotechnology companies and start-ups are valued in the billions of dollars without even a single market-approved cell therapy in their portfolios.
Immunotherapy and small biopharmaceutical companies as well as pharmaceutical giants all are scrambling to be first to market with one of these medicines. But some obstacles could hold up the progress of bringing these treatments to market: a host of logistical challenges to producing highly personalized, resource-heavy therapies on a commercial scale.

Figure 2: Two different types of cell therapies
The Inflection Point
The science of regenerative medicine has made tremendous progress over the past 10–15 years. Our increased understanding in areas such as stem-cell biology, human immune systems, and identification of disease-specific biomarkers all have contributed significantly to that progress. New molecular biology technologies — e.g., improved lenti-and adenoviral vectors, gene editing tools such as zinc-finger nucleases and the CRISPR/Cas9 platform — have opened doors to the development of novel gene-modified cellular therapies. However, the industry is missing bespoke production tools and equipment, manufacturing standards, and data as well as the necessary analytics to enable manufacturing on an industrial scale.
Although you could think of cell therapy production as a natural evolution of other types of pharmaceutical manufacturing, a few key aspects differ substantially,
making the manufacturing of cell therapies at times exponentially more challenging (Figure 2). Autologous (patient specific) therapies, present a particularly unique set of challenges in the way products are handled, manipulated, transported, and delivered to patients (Figure 3).
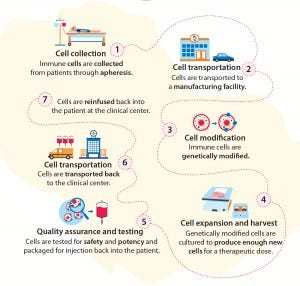
Figure 3: Autologous cell therapies — vein-to-vein individualized treatments
At scale, autologous therapies require complex parallel processing that pushes the boundaries of current biomanufacturing systems. Many related systems involve tools adopted from other industries such as cord and blood processing and banking, cell culture, and bioprocessing. To optimize manufacturing efficiencies, new and improved tools and technologies will need to be invented that are built for the specific purpose of cell therapy processing.
For autologous therapies, each patient represents an individual production batch and introduces his or her own unique biological differences to a process. So every autologous cell therapy batch — although similar in the equipment, reagents, and consumables used — will exhibit small biological variations that can affect results and thus requires of companies a deeper understanding about batch variability.
Autologous therapies may be advancing quickly to market, but we’ve yet to see a simple manufacturing process for them. A single cell batch may go through more than 10 steps in a “vein-to-vein” process that ends with returning final product to a clinical center for patient administration.
The regenerative medicine industry is grappling with a number of challenging questions:
How will we implement a robust and reproducible cell therapy manufacturing process that can run in parallel for the thousands of patients who require such personalized treatments?
How will we maintain chain of custody through such a highly complex process to ensure that each patient’s own cells will be administered to that patient alone?
How should we integrate data and analytics to make our processes smarter, more efficient, and more automated?
How can we keep up with demand for tools and technologies — and standardize their use — to keep pace with this rapidly progressing science?
Industrial-scale Cell Therapy Solutions
Even before biotech investments poured into the emerging field of cell therapy, GE recognized that industrialization challenges would need to be overcome. And although producing locomotives, jet engines, oil rigs, and wind turbines may not seem connected with cutting-edge clinical science, the common thread among the company’s businesses remains strong: It is a leading infrastructure provider to industrial markets.
This is not the first time that GE has translated its industrialization expertise to the world of medicine. For over 30 years, GE’s Life Sciences business has provided tools and technologies to help biotechnology and pharmaceutical companies manufacture biologics. Although GE borrows heavily from its industrial roots to help solve the complex challenges of cell therapy manufacturing, so too does it tap into world-leading innovation resources to design new and better ways to derisk and simplify cell manufacturing.
A few years ago, GE began introducing tools (e.g., the Xuri cell-expansion system) to fill gaps in production workflows. The company believes that working closely with those who are bringing cell therapies to market would be the most efficient and valuable way to continue improving its related technologies.
A Collaborative Innovation Center for Cell Therapy: CCRM is funded in part by the Canadian government’s Networks of Centres of Excellence program, the Province of Ontario, and leading academic and industry partners. It was created to support development and commercialization of regenerative medicines and associated enabling technologies, with a specific focus on cell and gene therapy. CCRM has built a strong network of academic researchers, leading companies, strategic investors, and entrepreneurs all seeking to accelerate translation of scientific discoveries into marketable products for patients. With a reputation for world-renowned expertise in regenerative medicine, this not-for-profit enterprise was built around an academic consortium that delivers a robust pipeline of discoveries and access to intellectual property (IP). CCRM is unique for bundling that IP into new applications and creating spin-off companies.
GE and CCRM soon realized that they should work together on the mutual goal of establishing world-class tools and technologies to help bring cell therapies to market. We wanted to take that challenge off the plates of cell therapy developers so that they could focus on the clinical aspects of the field without having to worry about how to make the products once they are approved. Both groups believe that leveraging complementary expertise to tackle the real-world challenges that the industry faces could have a dramatic and lasting impact on the field of regenerative medicine.
BrIdGE@CCRM
Our intention to create BridGE@CCRM was announced in January 2016. Scheduled to be up and running in late 2016, the center is meant to accelerate adoption of cell therapy technologies so that the regenerative medicine industry can bring such therapies to market as fast as possible. Managed jointly by GE and CCRM, the center will work with scientists, engineers, and clinicians in cell therapy, regenerative medicine, immunotherapy, bioengineering, and clinical trial management from both academic and industry settings. Together, we will home in on development and innovation of manufacturing technologies needed to collect, modify, and expand cells to dosing levels, quality-check them, and even transport them back to patients. The center will develop and intelligently implement both new and existing technologies, enabling manufacturing processes that can meet the commercial needs of cell-based medicines. A central theme of those processes will be the use of flexible, automated, and closed process technologies such as GE’s cell-expansion systems and Xcellerex bioreactors to produce new technologies that can manage data collection and management, quality control and analysis, raw materials, and cost reduction.

An operator uses GE’s Xuri cell-expansion system to grow modified cells up to an injectible batch size.
This collaborative model represents a new way of working to solve the complexities of an industry. We’re inviting development companies to bring their unique challenges to the center, where they can rapidly problem-solve with our support and input. Although competition between companies is fierce, we are all united in the common goal of bringing regenerative medicines to as many patients and as soon as possible. New tools and technologies will be crucial to cell therapy industrialization, helping to translate promising clinical opportunities into routinely applied clinical therapies with the potential to cure cancer and other life threatening and chronic diseases.
 The Right People in the Right Place Will Equal Industry-Moving Solutions: BridGE@CCRM will be strategically located in the active scientific community of Toronto, ON, where a concentrated and collaborative clinical infrastructure combines with the strong guidance of the internationally renowned CCRM. Nearly half of all Canadian stem cell researchers are based in Ontario, distinguishing the province as a prominent research hub (4). Toronto boasts the largest combined biomedical and biotechnology cluster in North America, with more than 11,000 principal investigators and technicians operating from nine teaching hospitals and 37 research institutions (5). The city also has the largest cluster of pharmaceutical companies in Canada, as well as the country’s largest concentration of research institutes, business incubators, and business support services. That makes it the densest geographical center for research in Canada. The MaRS Discovery District building, which will house this new facility, will contain one of the world’s largest clusters of translational activities in regenerative medicine (6). BridGE@CCRM brings together in one process development facility its own knowledge of the industry; therapy manufacturers with their cutting-edge science, real-world insights, and manufacturing challenges; and GE with its expertise in making industrialized manufacturing possible. Our vision is for this center to help solve specific challenges for companies who come to BridGE@CCRM and to commercialize nonproprietary innovations more broadly so that they will be available industrywide. Not only will those solutions enable scaled-out commercial cell therapy manufacturing, but they also will become industry standards, helping to drive this promising new frontier of medicine forward.
The Right People in the Right Place Will Equal Industry-Moving Solutions: BridGE@CCRM will be strategically located in the active scientific community of Toronto, ON, where a concentrated and collaborative clinical infrastructure combines with the strong guidance of the internationally renowned CCRM. Nearly half of all Canadian stem cell researchers are based in Ontario, distinguishing the province as a prominent research hub (4). Toronto boasts the largest combined biomedical and biotechnology cluster in North America, with more than 11,000 principal investigators and technicians operating from nine teaching hospitals and 37 research institutions (5). The city also has the largest cluster of pharmaceutical companies in Canada, as well as the country’s largest concentration of research institutes, business incubators, and business support services. That makes it the densest geographical center for research in Canada. The MaRS Discovery District building, which will house this new facility, will contain one of the world’s largest clusters of translational activities in regenerative medicine (6). BridGE@CCRM brings together in one process development facility its own knowledge of the industry; therapy manufacturers with their cutting-edge science, real-world insights, and manufacturing challenges; and GE with its expertise in making industrialized manufacturing possible. Our vision is for this center to help solve specific challenges for companies who come to BridGE@CCRM and to commercialize nonproprietary innovations more broadly so that they will be available industrywide. Not only will those solutions enable scaled-out commercial cell therapy manufacturing, but they also will become industry standards, helping to drive this promising new frontier of medicine forward.
References
1 Yuhas A. Cancer Researchers Claim ‘Extraordinary Results’ Using T-Cell Therapy. The Guardian 15 February 2016; www.theguardian.com/science/2016/feb/15/cancer-extraordinary-results-t-cell-therapy-research-clinical-trials.
2 Lentini L. Regenerative Medicine Could Be $20B Market in 15 Years. Fierce Biotech 23 April 2010; www.fiercebiotech.com/story/regenerative-medicine-could-be-20b-market-15-years/2010-04-23.
3 Annual Data Report on Gene and Cellular Therapies and the Regenerative Medicine Sector. Alliance for Regenerative Medicine: Washington, DC, 2015; http://alliancerm.org/sites/default/files/ARM_Annual_Report_2015_Web_Version_FINAL.pdf.
4 Canadian Asset Map for Stem Cell and Regenerative Medicine. International Biopharma Solutions Ltd.: Vancouver, BC, 2012; https://www.ic.gc.ca/eic/site/lsg-pdsv.nsf/vwapj/camscrmr_crcdcsmr_eng.pdf/$file/camscrmr_ crcdcsmr_eng.pdf.
5 Key Industry Sectors: Life Sciences. City of Toronto: Toronto, ON, 2014; www1.toronto.ca/wps/portal/contentonly?vgnextoid=2284c1b5c62c a310VgnVCM10000071d60f89RCRD&vgnextc hannel=401132d0b6d1e310VgnVCM10000071d 60f89RCRD.
6 Blocher P. Accelerating Commercialization: MaRS Helps Companies Shift into the Fast Lane. BioProcess Int. 6(2) 2008: 24–27.
Phil Vanek is general manager of cell therapy technologies at GE Healthcare’s Life Sciences business, 100 Results Way; 1-508-616-3078, [email protected].
You May Also Like





