Increased Speed to Market with an Emphasis on Quality
August 1, 2012

Speed to market has become a key factor of success in the biopharmaceutical industry, yet it can be difficult to achieve without compromising product quality. By seeking out products that are able to provide maximum efficiency while simultaneously maintaining first-rate quality, companies can not only get to market quicker, but also gain a competitive advantage. A new bag port line, manufactured by Value Plastics (VP), both addresses these concerns and tackles them head on by incorporating them into the product design.
Two aspects new to the industry and unique to the Bag Port Series are flow alignment ribs and parabolic entrance geometry. These features, respectively, promote a uniform flow by guiding the product into a consistent, rotational path while reducing flow separation from the wall. Design functionality and performance were validated in extensive testing and analysis, and then used in comparing the ports to other commercially available products.
Flow Test Results
To test volumetric flow rates, square-shoulder lead-in bag port models were measured against a VP design that included a parabolic-shaped lead-in. When evaluating the difference in performance, the curved entrance alone saw a volumetric flow rate of 8.37 gallons per minute (GPM), translating to a 26.6% increase over the model with a square entrance. This test was followed by comparing the same square model with VP’s final product design — a bag port that includes both flow alignment ribs and a parabolic lead-in. VP’s Bag Port Series yielded a 33.85% GPM increase in volumetric flow rate over the square model.
Computational fluid dynamics (CFD) analyses were also performed, in which the VP Bag Ports saw a 30.86% increase in volumetric flow rate when compared to other commercially available products. This can be attributed to the bag port’s curved entrance which promotes a consistent velocity profile being maintained throughout the flow path and a near elimination of flow separation. The result is a maximized use of space, allowing the largest amount of product to flow through the given inner diameter (Photo 1).
Photo 1:
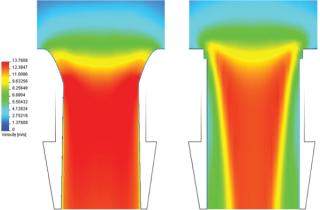
Photo 1: ()
Dramatic differences in flow characteristics were observed in additional testing. While the square-shouldered lead-in bag port generated an erratic turbulent flow (Photo 2), the parabolic lead-in produced a laminar straight flow. Incorporating the alignment ribs led to the eventual laminar rotating flow, providing the product with a more predictable and stable path.
Photo 2:

Photo 2: ()
Validation Test Results
Validation testing was performed on the two available bag port designs — barbed and ferrule. The features assessed included, but were not limited to: environmental stress-cracking resistance (ESCR); chemical compatibility; ESCR during extended use; barb fatigue; suction relief; hydrostatic pressure; side load; impact; leak; and gamma sterilization.
ESCR with alcohol testing was a key milestone to overcome in proving these components, validating that VP material will not crack during the designed life of the bag ports.
Value Plastics’ positive results in these tests are coupled with other critical features in biopharm applications such as an animal-free resin, USP Class VI certification, and guaranteed heat sealing and conformance with polyethylene bags. The Value Pharma™ resin used in these ports is unique in the market due to the combination of these qualifications.
About the Author
Author Details
Kyle Steele is product development engineer II at Value Plastics, a Nordson Company; 3325 S Timberline Rd, Fort Collins, CO 80525; 1-888-404-5837; www.valueplastics.com.
You May Also Like





