Application of Fabsorbent™ F1P HF, a Synthetic Ligand Adsorbent for Capture and Purification of a Single-Domain Antibody Fragment Expressed in
July 1, 2009

Figure 1:
Full-length antibody biopharmaceuticals continue to capture significant market due to their validated effectiveness as specific therapeutics. However, more recently it has been recognized that full-length antibodies may not always be necessary because antibody fragments can provide opportunity for new therapeutics with less complicated, higher-yielding production processes such as Escherichia coli and yeast microbial systems. For full-length antibodies, the downstream processing is facilitated by use of protein A–based affinity adsorbents for capture and initial purification; however there has been a lack of a similar specific adsorbent for the capture and initial purification of antibody fragments.
A synthetic ligand capable of binding to the light-chain binding site of antibodies was identified by screening of chemical combinatorial ligand libraries. The resulting adsorbent, Fabsorbent™ F1P HF, is effective for capture and initial purification of antibody fragments from a variety of sources.
Introduction
Fabsorbent™ F1P HF is a synthetic-ligand light-chain binding adsorbent that can be used as a superior alternative to Protein L for the capture and purification of antibody fragments including monovalent antibody fragments (e.g. Fab, scFv), engineered antibody variants, and single-domain antibodies. Fabsorbent F1P HF binds to both kappa (κ) and lambda (λ) light chains of IgG — unlike Protein L ligand adsorbent, which binds only to the κ light chain of the antibody molecule.
This application note describes the purification of a recombinant VL domain antibody, the smallest antigen-binding fragment expressed in E. coli antibody directly from cell lysate.
Materials and Methods
Recombinant VL domain antibody from cell culture lysate (adjusted to pH 8.0) was captured by chromatography on Fabsorbent F1P HF (1 × 2.5-cm column), equilibrated with 25 mM Tris–HCI (pH 8.0) buffer connected to a chromatography workstation. The residence time of the applied sample on the column was three minutes. Bound protein was eluted with 50 mM sodium citrate (pH 3.0) buffer. The column was cleaned with 0.5 M NaOH.
Fractions from the column were analyzed by SDS-PAGE using a 4–12% gradient Bis–Tris SDS-PAGE from Invitrogen and by gel-permeation chromatography using a TSK Gel Super SW3000 column from Tosoh Biosciences developed at a flow rate of 0.3 mL/min with detection at 280 nm. Total protein was determined with a Bradford assay, and host-cell proteins were measured using a commercial E. coli HCP ELISA Kit from Cygnus Technologies.
Results
Figure 1 illustrates the purification of recombinant VL domain antibody using Fabsorbent F1P HF. Most bound protein eluted as a sharp peak, with a small shoulder at the leading edge of the peak.
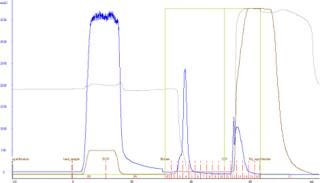
Figure 1: ()
Analysis of the column fractions by SDS-PAGE indicates that most host-cell related impurities flow through the Fabsorbent F1P HF column. The elution fraction contains concentrated target protein with some minor impurities (Figure 2). Purity of the eluted fraction was further assessed by analytical gel-permeation chromatography (Figure 3).
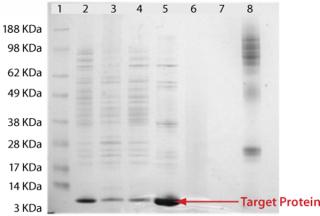
Figure 2: ()
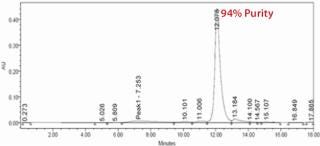
Figure 3: ()
The concentration of VL domain antibody in crude cell lysate was 0.5 mg/mL, representing 11% of the total protein applied onto the column. Recovery of the target protein in the elution fraction was 98% of the applied protein, indicating that Fabsorbent F1P HF provides high recoveries of the light-chain–containing antibody domain fragments. Additionally, a 98% HCP reduction was obtained in the elution fraction containing the target protein. The dynamic binding capacity of the adsorbent for this VL domain antibody is >9 g/L.
Conclusions
Fabsorbent F1P HF is an alternative to protein L–based adsorbents for the capture and purification of engineered light-chain–containing antibody domain fragments. It has superior binding capacity and provides good clearance of host-cell–related impurities, and elution of the bound protein by pH makes this adsorbent ideally suited for process scale applications.
You May Also Like





