Single-Use Technology for Aseptic Filling
July 1, 2008

Photo 1.
Biopharmaceutical processing has been greatly improved over the past 10 years with the introduction of single-use/disposable technology. There has been a landslide of disposable products introduced in the areas of filtration, mixing, sampling, and transferring solutions aseptically. It is common to find papers and studies that detail the efficiencies that disposables offer to the biopharmaceutical market. What about aseptic filling? More importantly, what about the cost of aseptic filling? PDC aseptic Filling Systems offers an answer.
The biopharmaceutical industry faces numerous cost pressures in today’s growing market. One such pressure point is aseptic filling. The question is how to reduce cost and increase production without compromising quality. Disposables are part of the answer, but many single-use products are expensive.
Some industry visionaries have begun to explore how “lean manufacturing” principles can help improve production efficiencies and reduce cost. Lean manufacturing practices and disposables fit hand-in-glove and represent a winning combination to achieve a more competitive production system.
PDC offers a new single-use breakthrough that will help companies reach a lean manufacturing profile. This unique technology is an aseptic filling system that offers disposability and multilayer cost savings. This new filling system offers all the usual benefits of disposables, but it goes further to generate unprecedented labor reduction, classified space savings, and production efficiencies — making it a vital asset in any aseptic filling operation.
The PDC filling system uses several innovative concepts that combine to offer fast, accurate, noncontact aseptic filling at a significantly reduced cost. An optional bag transfer table eliminates the need to lift filled bags as they are moved from the filler to the next operation. The transfer table allows operators to effortlessly roll each filled bag to the next production station.
The PDC filling machine uses a Coriolis mass flowmeter to measure and control fill volumes with results that are very accurate and repeatable. A disposable fill line is inserted through the Coriolis mass flowmeter. The measuring process is noncontact, with the solution flowing through the disposable fill line. The entire fill line — from its supply connection to its fill nozzle — is a single prefabricated, presterilized assembly. Fill lines are custom made to each customer’s specific requirements using Class VI materials (ADCF if required). The filling line includes a disposable nozzle at its fill end and is equipped with a disposable filter or aseptic connector at its supply end, depending on customer needs.
Photo 1.
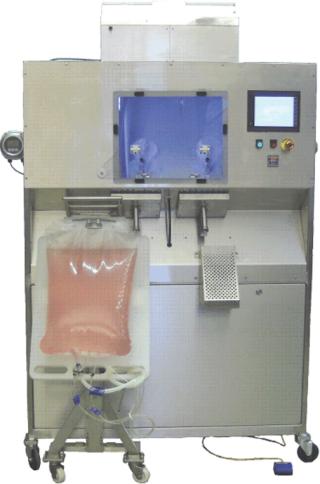
Photo 1. ()
A dual fill-head aseptic filling machine is slightly larger than a side-by-side refrigerator with wheels, making it portable. The filler has an integrated HEPA system that maintains an ISO 5 environment around the fill nozzle with positive pressure and laminar flow. An integrated on-board particle counter continuously monitors the fill chamber environment for ISO 5 compliance. An RF tube sealer automatically seals each bag’s fill tube after the bag has been filled and before the tube exits the ISO 5 environment.
The PDC filling machine is versatile, offering the capability to aseptically fill bags from 100 mL to 1,000 L in volume. Available options allow filling of smaller bags, containers, and bottles. The disposable fill line can be changed in about 10 minutes. Turnaround time can be as short as an hour between batch fills. The single-use fill line system eliminates the need for CIP SIP cleaning. For example, the same aseptic filling machine can be used to fill 5-L bags on one shift and then fill 200-L drums the next. The possibility of cross contamination is essentially eliminated because the entire fill line is replaced with each new batch. A typical fill line is about four meters long. So the total disposable waste per batch is four meters of plastic tubing.
Photo 2.
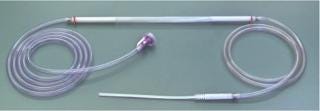
Photo 2. ()
To fill a bag or container, an operator inserts the fill tube into the PDC aseptic Filler system. Once a fill tube is inserted into the filler, the rest is automatic. The filling machine automatically fills the container in an ISO 5 environment and seals the fill tube after the fill. The system is fully validated and in operation in the United States and Europe.
The PDC filling system reduces needed classified fill space as well as the labor required to fill bags and the cost of bags when compared with manifold filling operations. It’s no longer necessary to purchase a manifold only to dispose of its backbone and residual solution. In other words, you don’t have to purchase the grapevine to get the grape.
For situations in which each bag has a capsule filter and aseptic connector, they can be eliminated when the filler is used to fill a bag. In case studies replacing manual filling through capsule filters or manifold filling, the payback period has been as short as seven months.
Filling bottles or bags with the PDC filling system eliminates operator interaction from the filling process. The fill volume is automatically batched by the Coriolis mass flowmeter, with operators isolated from the fill area. Sight filling and weigh scaling are eliminated with the filler.
PDC Aseptic Filling systems for bottles or bags are also important business tools as the biopharmaceutical industry strives to increase filling efficiency and reduce costs without compromising quality.

Photo 2.
You May Also Like





