- Sponsored Content
Continuous Processing and Integrated Facilities
August 11, 2016
Sponsored by Finesse
 Single-use technology has transformed the biomanufacturing industry, because single-use products provide significant savings over stainless steel infrastructure. Single-use technologies also create flexibility for biomanufacturers, enabling them to move from single-product processes to multiproduct facilities. As revolutionary and transformative as single-use technologies have been, the industry is on the cusp of another major shift, which comes with its own impressive benefits, but with some challenges as well.
Single-use technology has transformed the biomanufacturing industry, because single-use products provide significant savings over stainless steel infrastructure. Single-use technologies also create flexibility for biomanufacturers, enabling them to move from single-product processes to multiproduct facilities. As revolutionary and transformative as single-use technologies have been, the industry is on the cusp of another major shift, which comes with its own impressive benefits, but with some challenges as well.
Continuous and Batch Processing
Continuous processing has been used successfully for many years in a number of manufacturing industries such as in the production of food, chemicals, and steel. When applied to biomanufacturing and combined with single-use technology, continuous processing can offer much higher throughput and increased flexibility.
Batch processing has been the logical method in biomanufacturing for regulatory compliance and quality control. The batch method establishes clear boundaries, with unit operations delineated by transition points, thereby allowing an operation to be regulated in an organized manner.
By contrast, in continuous processing, material can run uninterrupted from cell cultivation to the final polishing step. For an industry that is accustomed to monitoring quality at every stage, however, this raises numerous issues:
If a product proceeds continuously through all operations, how do you define a “batch”?
If the end product fails, how do you identify and document where the failure occurred?
If a portion of the final product is found to be suspect, can the previous product be trusted?

Islands of Data
With batch operations, identifying a bad lot is straightforward. Continuous processing, although it promises higher efficiency and increased productivity, poses a new challenge for reliable lot release. Because continuous processing lacks the intermediate cut-off points featured in batch processing, in-line measurement must be included so that the quality of proteins and antibodies can be identified at certain points in a process. Analyzers that can perform this function are not widely available, and the devices that can be obtained often are not fast enough when delivering data. The result, therefore, can be an inability to pinpoint where in the process a contamination or quality issue occurred.
Another essential consideration in moving to continuous processing is addressing the connection between the various processes involved. Whereas batch processing focuses on individual unit operations of a batch process, continuous processing, to be successful, must connect the “islands of data” with some method of communication. The interdependence between measurement and real-time actions requires global process optimization. A vital tool for this communication is open platform communications (OPC). Microsoft developed the OPC specification in the mid-1990s for use in manufacturing automation applications to aid interoperability. The standards define consistent methods for accessing field data from plant floor devices. In biomanufacturing, OPC can provide connection between various controllers (e.g., Siemens or Emerson DeltaV), data storage in one large repository, unit steps to interact with one another, and off-line analytics.
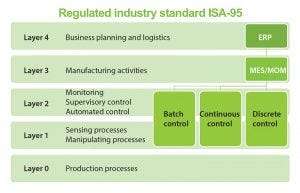
Figure 1: Regulated industry standard ISA-95
Manufacturing Execution Systems
A facility also can add a manufacturing execution system (MES). In an MES, the plant network architecture consists of several layers:
Business layer for planning a business and logistics.
MES layer, which contains the master recipe and the electronic records of each process step; operates the automation islands in synchronicity and accounts for transition steps to complete the process.
Harmonization layer, which provides open connectivity between controllers and the MES layer, tying the islands of data together.
Control layer, which controls each unit operation and gathers process data within a repository (called a historian database).
Measurement layer, which provides process parameter information to the control and MES systems.
The MES layer is vital to integrate the data and the transition between operations. This integrated approach allows a facility’s information systems and actions to function as a whole. If a problem is detected in one part of the process, the process can be stopped before it consumes a significant amount of materials. For example, if a protein is found to be of low quality, a facility could avoid wasting the chromatography resins to purify it.
The Importance of Flexibility
Integrated facilities provide flexibility and (in the case of multiproduct facilities) the ability to reconfigure a process train, depending on the campaign of the molecule being produced. The ability to mix and match equipment is extremely important, because certain vendors may have better solutions for a particular process step. This flexibility also has benefits for supply-chain integrity and price. If a vendor has a quality or supply issue, or if it has a price elevation over time, then the option to add a new supplier can provide stability or reduce costs.
Multiproduct facilities must ensure that a process train reconfiguration is as fast and easy as possible. Because two products may have very different titers, variables such as equipment selection, the order in which equipment is used, and the number of steps or cycles per piece of equipment should be easily reconfigurable.
The traditional MES system is designed for one product and one process, with the goal of maximizing yield at the lowest possible cost. Multiproduct facilities must be able to rapidly respond to a product (and thus, process) change using existing equipment. For example:
• A facility that produces flu vaccines might have to reconfigure their product mix/volume for a given year, depending on the vectors the CDC issues that year.
• A company that has various orphan drugs in its pipeline might need to quickly ramp up production with a sudden change in response to demand, the population, or a new approval.
The top MES layer can allow a facility to quickly transition between products in a validated manner, because it enables a facility to take unit operations in and out of use without losing integration.
Conclusion
With many companies building new plants based on current technology trends, technology clearly is moving toward more flexible, more efficient production, and ultimately more effective therapeutics. Continuous processing, combined with integrated facilities, promises higher efficiency and increased productivity.
Barbara Paldus, PhD, is CEO and cofounder of Finesse Solutions, Inc., 3501 Leonard Court , Santa Clara, CA 95054; 1-650-533-8432.
You May Also Like





