- Sponsored Content
Implementation of Single-Use Miniature Bioreactors to Support Intensified Cell Culture: Using Functional Performance Indicators to Assess a Small‑Scale Model
March 3, 2020
Sponsored by Sartorius
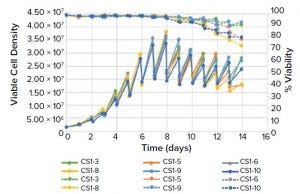
Figure 1a: Viable cell density (VCD) and viability of Chinese hamster ovary (CHO) cells cultured using cell settling in minibioreactors
Changes to bioprocessing in the biopharmaceutical industry are driven by the need for increased speed, lower cost of goods (CoG), and greater flexibility (1). To meet these challenges, the industry is adopting strategies that include intensified processing. During the initial stages of intensified processing, it is essential to identify the most productive and/or stable clones for use before starting pilot-scale studies. That requires screening large numbers of clones and then further testing the most promising ones in benchtop bioreactors. The need to perform large numbers of time-consuming, resource-intensive experiments has led to development of micro- and mini-scale bioreactor systems that offer a small-scale, high-throughput (HT) solution for accelerating clone/media selection and process development.
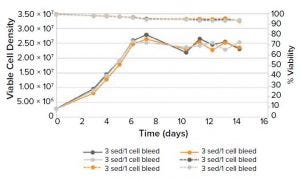
Figure 1b: Viable cell density (VCD) and viability of CHO cells cultured using centrifugation in minibioreactors
According to a 2018 survey (2), over 80% of the biopharmaceutical industry uses Sartorius Stedim Biotech’s (SSB) ambr micro- and ambr minibioreactors as mimics of benchtop bioreactors for fed-batch and batch cell culture. To date, more than 400 ambr 15 and 200 ambr 250 systems have been sold worldwide. Industry experts envision that this automated system will dominate replacement of standard laboratory-scale glass reactors for process development and characterization studies over the next decade (3).
Thus, it makes sense to investigate the effects of perfusion culture on viable cell density (VCD), cell viability, and product titer performance with ambr minibioreactors to determine whether they are suitable models for intensified processing. Successful process intensification requires cell lines that can be cultured in perfusion conditions. The ambr 15 cell culture (cc) system has proven to be successful for clone selection, performing comparably to benchtop bioreactors (4). This system combines 24 or 48 10–15-mL working-volume single-use bioreactors containing stirred impellers. The bioreactors are integrated with an automated cell culture station with dedicated control and analysis software.
For effective scale-up of intensified processes, process conditions need to be modeled accurately. In 2018, SSB introduced the ambr 250 HT perfusion system for this application. Based on the ambr 250 HT system, developed in 2013 in collaboration with Merck (5), the ambr 250 HT perfusion system uses up to 24 single-use perfusion stirred bioreactors (100–250 mL working volume), with associated single-use perfusion components such as hollow-fiber filters for cell retention, all controlled by an automated cell culture station. The ambr minibioreactors have similar geometries to those of larger stirred-tank bioreactors, providing helpful process intensification conditions for seamless transfer to manufacturing scale.
Materials and Methods
Perfusion Cell Culture for Clone Selection — Cell Settling: To determine whether the ambr 15 cell culture system could be used to mimic larger scale perfusion cell culture mimic, a cell-settling method was investigated. Cellca CHO cells expressing an antibody were cultured in Cellca Chinese hamster ovary (CHO) media (10 mL) in minibioreactors (n = 3) using simulated perfusion conditions (37 °C, stirring speed 300 rpm) for 14 days. When cells reached a density of >20 × 106/mL, the culture station was switched off to allow them to settle at the bottom of the vessel (at different settling times from 20 to 40 minutes to determine an optimal cell settling time). After cells had settled, a medium exchange of one vessel volume/day (VVD, 3.33 mL) was performed, resulting in a cultivation cell density of 20 × 106/mL. The number of times the cell culture station was switched off per day also varied from one to three times to determine the optimal number of settlings per day.
Cell Centrifugation: A centrifugation method also was investigated to determine whether the ambr 15 cell culture system could be used as a perfusion mimic. Cellca CHO cells expressing an antibody were cultured in Cellca CHO media (10 mL) in minibioreactors (n = 6) using simulated perfusion conditions (37 °C, stirring speed 300 rpm) for 14 days. The culture station was switched off daily when cells reached a density of >20 × 106/mL, and bioreactors were centrifuged using specially adapted centrifuge inserts. The design of the inserts caused pellets to form away from sensor elements in the bioreactor vessels, ensuring robust ongoing functionality. A daily media exchange of 1 VVD (3.33 mL) was performed, removing media and replacing it with fresh media to achieve 20 × 106/mL.
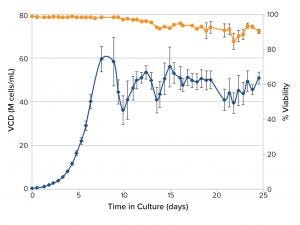
Figure 2: Viable cell density (VCD) and viability of CHO cells cultured for 25 days in miniature perfusion bioreactors
Perfusion Cell Culture for Process Development — Cell Culture at 50 × 106 cells/mL: To determine whether ambr 250 HT perfusion bioreactors could sustain consistent growth up to 50 × 106 cells/mL, Cellca CHO cells expressing an antibody were cultured in Cellca CHO media (100 mL) in minibioreactors (n = 18) using perfusion conditions (37 °C, stirring speed 300 rpm) for seven days. The cell culture station was integrated with a Bioprofile FLEX2 automated cell culture analyzer (Nova Biomedical) with an external sample module (ESM), which managed and transferred a sample volume (0.4–0.7 mL) to monitor for VCD and cell viability. The data were transferred back to the cell culture station in real time for automated feedback control of cell culture bleed and media replacement when the FLEX2 measured a VCD greater than 50 × 106 cells/mL.
To assess whether the minibioreactors could maintain growth up to 50 × 106 cells/mL for a longer time, Cellca CHO cells expressing an antibody in Cellca CHO media (100 mL) were cultured in mini bioreactors (n = 8) using the same perfusion conditions as above for 21 days. The culture was left for a longer time to determine the maximum period for a perfusion run. The VCD was measured either on-line or off-line using a Cedex cell analyzer (Roche), and a fully automated cell culture bleed and media replacement was performed when the VCD was greater than 50 × 106 cells/mL.
To establish whether the minibioreactor and fully automated VCD control could maintain growth of up to 50 × 106 cells/mL for longer times, Cellca CHO cells expressing an antibody were cultured in Cellca CHO media (100 mL) in minibioreactors
(n = 20) using the same perfusion conditions as before for 21 days. An automated daily cell culture bleed and media replacement were performed when the VCD was greater than 50 × 106 cells/mL, assessed by the integrated FLEX2.
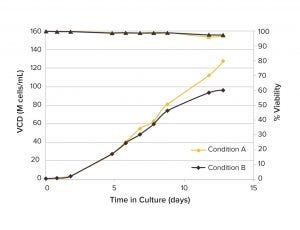
Figure 3: Viable cell density (VCD) and viability of CHO cells cultured for 14 days in miniature perfusion bioreactors
Cell Culture at 100 × 106 cells/mL: To determine whether the minibioreactor allows growth greater than 100 × 106 cells/mL, Cellca CHO-S cells (nonexpressing) were cultured using two different media conditions (100 mL) in ambr 250 vessels (n = 12 for each medium) under perfusion conditions (37 °C, stirring speed 300 rpm) for 14 days and a maximum perfusion media flow 4 VVD, 9.5 g/L glucose. The VCD was measured either on-line or off-line using a Cedex system. An automated cell culture bleed and media replacement was performed when the VCD was greater than 100 × 106 cells/mL.
Comparison with Benchtop Bioreactors: To compare the VCD, cell viability, and titer performance of ambr 250 HT perfusion bioreactors with those of benchtop bioreactors, a biopharmaceutical partner cultured a cell line expressing a coagulation factor using proprietary media and culture conditions over 30 days in minibioreactors (n = 2) and a 5-L benchtop bioreactor (n = 1) for VCD; and cell viability measurement and ambr 250 HT perfusion bioreactors (n = 4) and 5-L benchtop bioreactors (n = 2) for product titer. Both ran in alternating tangential flow (ATF) mode. Crossflow and dilution rates used were within typical operating ranges. Automated bioreactor cell culture bleeding and media replacement were based on daily cell counts to keep VCD within reference process target range. Additionally, four quality attributes (designated A, B, C, and D) were tested each week from the miniature vessels and 5-L bioreactors during the three-week run.
Comparison with Pilot-Scale Bioreactors: To compare the VCD performance of ambr 250 HT perfusion with that of a pilot-scale bioreactor, a biopharmaceutical partner cultured a proprietary cell line using proprietary media and culture conditions over 24 days in the minibioreactors (n = 2). Crossflow and dilution rates were within typical operating ranges. Automated bioreactor media bleeding and replacement were based on a set target VCD range. The VCD results were compared with historical VCD data from a 100-L bioreactor.
Results and Discussion
Cell Settling and Centrifugation for Perfusion Culture: Cells were maintained at a VCD in the range of 25 × 106 cells/mL (Figure 1, left) and achieved titers of approximately 1 g/L/d from days six to 14, generating a cumulative titer of greater than 7 g/L using the ambr 15 cc bioreactors and an optimum cell settling regime of 40 min, with three settles/day. All replicates showed good consistency.
Using ambr 15 bioreactors with daily centrifugation and media exchange, the cultivations had a VCD in the range of 25–30 × 106 cells/mL (Figure 1, right). All replicates showed good consistency and achieved titers of about 1.5 g/L/d from days six to 14, generating a cumulative titer of >15 g/L.
These results show that cell settling and cell centrifugation are suitable as simulated perfusion protocols. Both methods produced differences in absolute titer, but either technique could help accelerate clone and media selection for intensified processes.
Intensified Process Development — Cell Culture at 50 × 106 cells/mL: Using miniature perfusion bioreactors in an automated cell culture system integrated with the FLEX2 system, cells were maintained at a VCD in the range 50 × 106 cells/mL. Cell viability of all replicates was consistently high over seven days and showed some fluctuation in replicates (Figure 2) over 24 days. Results indicate that the combination of integrated FLEX2 VCD measurement and ambr 250 HT perfusion system for automated bleeding can support perfusion culture for more than three weeks.
The miniature perfusion bioreactor process exceeded the 21-day target and maintained cells at a VCD in the range of 50 × 106 cells/mL for 34 days (figure not shown). Consistently high viabilities (98.9%), indicate that the minibioreactor can support extended perfusion culture at this level of cell density.
Cell Culture at 100 × 106 cells/mL: A VCD of 125 × 106 cells/mL was achieved using media condition A and maintained good viability over 10 days (Figure 3) in the miniature perfusion bioreactors. But with media condition B, the VCD began to decrease with the decrease in glucose concentration. Results show that the ambr 250 HT perfusion bioreactor can maintain high cell densities and also can enable users to distinguish the effects of alternative media conditions. That makes this system suitable as a model for process media assessment in high-density perfusion cell cultures.
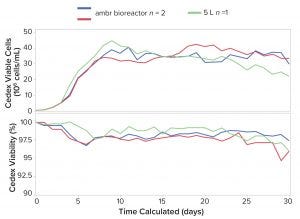
Figure 4: Comparison of viable cell density (VCD) and viability of CHO cells cultured for 30 days in miniature perfusion bioreactors and 5-L benchtop bioreactors
Comparison with Benchtop Bioreactors: Other results showed that cell growth and viability (Figure 4) as well as product titer (data not shown) were consistent among miniature perfusion bioreactor replicates and were comparable to those seen in the 5-L benchtop bioreactors for up to 30 days. Additionally, the four quality attributes tested showed good consistency between both bioreactor types, indicating that product quality in ambr 250 HT perfusion bioreactors is representative of larger scale perfusion cultures.
The VCD profile results from the miniature perfusion bioreactors and 100-L bioreactors were comparable (Figure 5). Also, product titer and metabolite profiles also matched well in the two bioreactor types (data not shown), indicating that the ambr 250 HT perfusion bioreactor also could be used to model manufacturing-scale perfusion cultures.
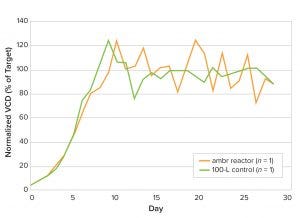
Figure 5: Comparison of viable cell density (VCD) from cells cultured in miniature perfusion bioreactors and 100-L bioreactors
A Significant Enabling Technology
Studies have shown that ambr single-use minibioreactor technology can be used to model perfusion cell culture for up to 34 days to maintain VCDs from 25 to 125 × 106 cells with cell viabilities of up to 98%. When compared with 5-L and 100-L bioreactors, the VCD, viability, product titer, and product quality match well with results from the ambr 250 HT perfusion bioreactor, indicating that this minibioreactor could be used as a scale-up model to achieve intensified cell cultures ≥125 × 106 cells/mL and scalability ≤100 L.
Using this single-use small-scale technology in place of benchtop bioreactors minimizes downtime between experiments, reportedly requiring up to 87% less medium than benchtop bioreactors and reducing hands-on time by ~50%. Thus, the ambr 250 HT perfusion system is a significant enabling technology for reducing complexity and set-up time while increasing experimental capacity and data consistency by using a fully automated parallel cell culture station. This will increase cost effectiveness attributable to media and labor cost savings as well as improve performance of larger studies and perfusion design of experiments (DoE).
In summary, ambr technologies from SSB enable the biopharmaceutical industry to accelerate implementation of intensified and perfusion cell culture at scale, delivering significant reductions in development timelines and CoGs of biologics manufacturing.
References
1 Biophorum Operations Group (BPOG). Biomanufacturing Technology Roadmap, 2017; https://www.biophorum.com/wp-content/uploads/2017/11/Biomanufacturing-Technology-Roadmap.pdf.
2 Survey: Do You Have Mini-Bioreactors (Less Than 1 L Total Volume) at Your Site? Aspen XChange, 25 September 2018; https://aspenxchange.com/2019/08/07/26305.
3 Pollard D, et al. Automated Disposable Small-Scale Bioreactor for High-Throughput Process Development: Implementation of the 24-bioreactor Array. Pharm. Bioprocess 3(3) 2015: 185–197.
4 Lewis G, et al. Novel Automated Micro-Scale Bioreactor Technology: A Qualitative and Quantitative Mimic for Early Process Development. Bioprocess. J. 9(1) 2010: 22–25.
5 Bareither R, et al. Automated Disposable Small Scale Reactor for High Throughput Bioprocess Development: A Proof of Concept Study. Biotech. Bioeng. 110(12) 2013: 3126–3138.
Corresponding author Ian Ransome is the head of product management, ambr systems, at Sartorius Stedim Biotech, Royston, UK; [email protected].
You May Also Like





