Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
July 1, 2009

Figure 1:
This protein proved to be “difficult to express” in both mammalian (CHO) and yeast (Pichia pastoris) expression platforms. In CHO cells, expression levels were very low, <50 mg/L/day, and the product was subject to proteolysis and aberrant O-glycosylation. In yeast, expression levels were highly variable, the product was heavily mannosylated, and it was also subject to extensive proteolysis. Glycosylation is known to be immaterial to either the pharmacological or pharmacokinetic properties of this protein. Therefore, mammalian or yeast-cell expression is inappropriate because these hosts create troublesome contaminants of glycosylated products, not to mention the degraded target protein as well.
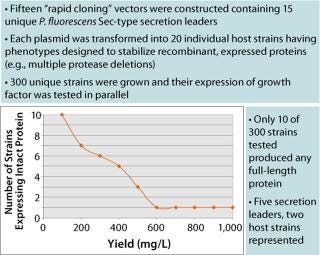
The Pfēnex team designed a strategy to address the expression of this proteolytically sensitive, disulfide-bonded protein. Multiple sec-type secretion leaders were fused to the coding sequence to target the growth factor to the periplasmic space. Once constructed, these expression plasmids were inserted into multiple host strains in which individual and multiple annotated proteases had been deleted. In all, 300 completely unique expression strains were constructed, cultured, and analyzed in less than four weeks!
All of the steps in this process were conducted using 96-well plates and robotically assisted. This novel, high-throughput, parallel strain construction and screening work process has been made possible with the “Pfēnex Expression Technology™ Toolbox,” an off-the-shelf, seamlessly integrated collection of expression plasmids (containing multiple and varied transcription, translation, and secretion signals) and engineered host strains (expressing phenotypes designed to help proteins fold or maintain their structural integrity).
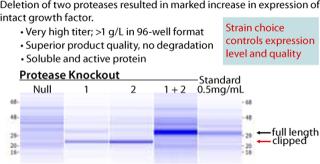
Figure 1 shows that of the 300 strains constructed, only 10 expressed any measurable growth factor (SDS-CGE), an astonishing 3%. This observation thoroughly validates this high-throughput parallel approach to expression strain construction and screening. Figure 2 shows that the best-expressing strain, producing >1 g/L at the 96-well scale, had deletions of two proteases that when singly deleted showed marginal expression of the growth factor. When those cells are grown in a fermentor, we anticipate a further three- to five-fold increase in titer. These results powerfully demonstrate the utility of the Pfēnex Expression Technology™ toolbox approach for expression strain development.
You May Also Like