Recombinant Antibody Technology: Its Criticality to Past and Future Biopharmaceutical SuccessRecombinant Antibody Technology: Its Criticality to Past and Future Biopharmaceutical Success
April 17, 2024
Recombinant antibody technology addressed many problems associated with hybridoma-based platforms for biologics production, opening the door to new classes of drugs. Today, recombinant technology still contributes to the discovery and development of antibody-based therapeutics while helping to improve the performance of other biological modalities.
Early monoclonal antibody (mAb) therapies were developed using hybridoma platforms, which involve immunization of animal subjects with target antigens or immunogens. Such technologies were beset not only by ethical issues, but also by batch-to-batch variability, antibody heterogeneity, diminished antibody productivity, limited scalability, and high costs. Moreover, hybridoma-based processes took multiple months from immunization to establishment of specific clones and mAb production.
On the other hand, recombinant antibodies are generated from host cell lines using recombinant DNA technology. This approach greatly reduces reliance on animal experimentation while yielding large quantities of antibody products quickly — within just a few weeks, in most cases. Based on known DNA sequences that can be replicated exactly, recombinant antibody production is highly controllable, reproducible, and consistent. Moreover, recombinant antibodies offer advantages including capabilities for targeting hybridoma-refractory antigens and amenability to engineering.
Many Advantages from Engineering
Recombinant antibody engineering provides many opportunities for creating molecules with attributes that are optimal for treating specific diseases. An antibody’s fragment crystallizable (Fc) region can be modified to prevent initiation of undesired responses, and tiny variable regions can be designed to increase target-binding effectiveness. Therapeutic antibodies that scientists developed in mice or other animals also can be humanized to reduce immunogenicity.
Decades of experience now inspire confidence about which genetic changes can realize specific benefits. As artificial intelligence (AI) algorithms advance, that level of predictability will only increase.
Figure 1: Technical workflow for Sino Biological’s high-throughput recombinant production platform
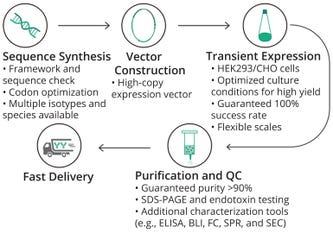
BLI = biolayer interferometry CHO = Chinese hamster ovary
ELISA = enzyme-linked immunosorbent assay FC = flow cytometry
HEK = human embryonic kidney SEC = size-exclusion chromatography
SDS-PAGE = sodium dodecyl sulfate–polyacrylamide gel electrophoresis SPR = surface plasmon resonance QC = quality control
In fact, many drug developers are leveraging AI technologies to generate and analyze potential therapeutics from antibody sequences before entering production stages. Then, scientists can apply high-throughput production and characterization methods to validate AI-based predictions in a laboratory setting, helping to accelerate antibody discovery and development. Meanwhile, platform production processes facilitate rapid scale-up to meet clinical and commercial demand.
Fragment Understanding
An important advantage of recombinant antibody technology is its versatility. That enables scientists to explore and understand many “flavors” of mAbs — and possibilities are limited primarily by our imaginations. Recent years have witnessed the rise of engineered antibody fragments such as antigen-binding fragment (Fab), single-chain fragment variable (scFv), and single-domain heavy-chain–only antibody fragment (VHH) formats.
Conventional mAbs have certain limitations in clinical applications. Immunoglobulin G (IgG) molecules are relatively large (~150 kDa), which can prevent them from penetrating effectively into tissues. In addition, the presence of the Fc region in IgG molecules can mediate bystander activation of a patient’s immune system. However, antibody fragments retain the targeting specificity of intact mAbs and possess superior properties for many therapeutic and diagnostic applications. Because of their small sizes, antibody fragments can enhance tissue penetration, enable binding to traditionally inaccessible epitopes, and reduce immunogenicity. Fragments also can provide structural flexibility, facilitate production and engineering, and serve as building blocks for novel constructs.
Production and purification of antibody fragments require extensive expertise, especially because of recent diversification in formats and increases in design complexity. Several aspects should be considered carefully, including design strategies, upstream production optimization (e.g., to increase productivity while suppressing impurity generation), and downstream processing requirements.
Modifications for Different Applications
Although antibodies for research, diagnostics, and therapeutics all must exhibit high binding affinity and specificity, specific applications have distinct requirements. When biomedical researchers require antibodies that can penetrate different tissues to assess various molecular and behavioral characteristics, antibody fragments hold advantages over full-length IgGs. For diagnostics, engineered multivalent antibodies with enhanced antigen-binding avidity are promising agents. For therapeutic applications, recombinant antibodies can be modified in myriad ways: to prevent cross-reactivity, to increase targeting of effector cells, to enable site-specific conjugation to small-molecule payloads, and to achieve many other desired characteristics.
Unlocking Significant Potential
Although the therapeutic possibilities presented by cell and gene therapies and other novel modalities are generating much excitement, recombinant antibodies still have significant potential going forward. Because they enable targeted treatment with minimal side effects, they will continue to play critical roles in advancing treatments for cancers as well as autoimmune, metabolic, and infectious diseases. Beyond traditional mAbs are bispecifics, antibody fragments, antibody–drug conjugates (ADCs), and other next-generation antibody therapeutics with novel functionalities that could enhance targeting capabilities and thus increase efficacy.
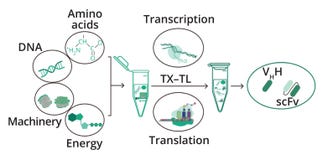
Figure 2: Cell-free synthesis of antibody fragments; scFv = single-chain fragment variable, VHH = single-chain heavy-chain–only antibody fragment, TX–TL = cell-free transcription–translation reactions
Building Recombinant Antibody Expertise
Sino Biological has leveraged recombinant technology for over 15 years, not only to generate catalog protein and antibody products, but also to build the company’s contract development and manufacturing business. To meet diverse customer needs, Sino Biological has established comprehensive expression capabilities, including multiple cell-based systems (e.g., platforms based on mammalian cells and insect cells) and fast, efficient cell-free platforms. Customers can benefit from Sino Biological’s many years of experience with not only IgGs, but also dimeric IgAs, multivalent IgMs (pentameric and hexameric), antibody fragments, and bispecifics.
Mammalian-Cell Expression: For human research or clinical studies, mammalian cells provide several advantages for antibody production. Such cells can express antibodies and other molecules that mimic naturally occurring proteins in humans, including requisite posttranslational modifications. Sino Biological’s optimized mammalian-cell platforms use proprietary culture media, transfection reagents, expression vectors, supplements, and boosters. The high-throughput platform can work on more than 1,000 projects simultaneously from gene synthesis to production of recombinant antibodies for discovery and screening purposes (Figure 1). For example, customers can receive more than 1,000 different antibodies in just two weeks. Sino Biological also can produce antibodies in quantities ranging from micrograms to kilograms, ensuring that customers worldwide receive products with high consistency and quality at a desired quantity.
Sino Biological’s Center for Bioprocessing (C4B) in Houston, TX

Cell-Free Protein Synthesis: Cell-free systems enable rapid in vitro production of antibodies and other proteins — typically in hours rather than days or weeks. Such systems can produce difficult-to-express proteins, with open-processing conditions that facilitate direct manipulation of the chemical environment and that hold potential for high throughput. Sino Biological’s cell-free platform has proven itself with successful production of proteins and antibodies that are difficult to express in other systems, and the platform’s short lead times help to accelerate customers’ research and development processes. For example, the cell-free platform can generate antibody fragments such as VHHs and scFvs in three hours, and a one-day purification process yields highly pure preparations of antibodies with binding activity comparable to that of antibody fragments expressed in mammalian cells (Figure 2).
A New Facility: In October 2023, Sino Biological announced the formal opening of its new Center for Bioprocessing (C4B) in Houston, TX. The launch marks a significant milestone in the company’s global expansion. The center specializes in contract research services, including development and manufacture of custom recombinant antibodies and proteins. The team is committed to delivering high-quality products and is eager to partner with researchers and industry leaders worldwide to forge a brighter future in the life sciences.
References
1 Geigert J. The Challenge of CMC Regulatory Compliance for Biopharmaceuticals (Fourth Edition). Springer: Cham, Switzerland, 2023; https://doi.org/10.1007/978-3-031-31909-9.
2 Auclair J, Rathore AS. Intact Mass Analysis of Biopharmaceuticals as Its Own Unique Application. LCGC North Amer. 39(11) 2021: 548–553; https://www.chromatographyonline.com/view/intact-mass-analysis-of-biopharmaceuticals-as-its-own-unique-application.
3 Staub A, et al. Intact Protein Analysis in the Biopharmaceutical Field. J. Pharm. Biomed. Anal. 55(4) 2011: 810–822; https://doi.org/10.1016/j.jpba.2011.01.031.
4 Mans J, et al. The Use of Mass Spectrometry in Therapeutic Protein Biologics License Applications: A Retrospective Review Revisited. J. Am. Soc. Mass Spec. 34(11) 2023: 2575–2584; https://doi.org/10.1021/jasms.3c00286.
5 Vessely C, Bussineau C. QbD in Biopharmaceutical Manufacturing and Biosimilar Development. Biosimilars: Regulatory, Clinical, and Biopharmaceutical Development. Gutka HJ, Yang H, Kakar S, Eds. Springer: Cham, Switzerland, 2018, 187–219; https://doi.org/10.1007/978-3-319-99680-6.
6 Vandekerckhove K, Reeve R. Principles of Analytical Similarity Assessment. Biosimilars: Regulatory, Clinical, and Biopharmaceutical Development. Gutka HJ, Yang H,
Kakar S, Eds. Springer: Cham, Switzerland, 2018, 261–303; https://doi.org/10.1007/978-3-319-99680-6.
7 Sullivan PM, DiGrazia LM. Analytic Characterization of Biosimilars. Amer. J. Health Sys. Pharm. 74(8) 2017: 568–579; https://doi.org/10.2146/ajhp150971.
Further Reading
Kaltashov IA, Wang S, Wang G. Mass Spectrometry in Biopharmaceutical Analysis. Walter de Gruyter GmbH: Boston, MA, 2022; https://doi.org/10.1515/9783110546187.
Rathore AS, Mhatre R, Eds. Quality by Design for Biopharmaceuticals: Principles and Case Studies. John Wiley & Sons: Hoboken, NJ, 2011.
Houde DJ, Berkowitz SA, Eds. Biophysical Characterization of Proteins in Developing Biopharmaceuticals. Elsevier: Amsterdam, Netherlands, 2019.
Kaur H, Reusch D, Eds. Monoclonal Antibodies: Physicochemical Analysis. Elsevier: Amsterdam, Netherlands, 2021.
Suranjana Sen, PhD, is a technical account manager at Sino Biological US Inc.; 1-215-583-7898; [email protected]; https://www.sinobiological.com. This content has been created in collaboration with Pharma’s Almanac.
You May Also Like





