Figure 1:
SterilEnz®-II/G connectors and SterilEnz Samplers represent a novel design improvement when compared with common fittings and fluid injection sites. This article briefly discusses the features, benefits, and technical aspects surrounding the design and integrity testing of the SterilEnz product line as well as advantages over their predecessors.
SterilEnz-II/G Flange Connectors and Samplers
Each SterilEnz-II/G connector is hermetically sealed inside a rugged overwrap pouch (Figure 1). The connector is fitted with a pre-attached, medical grade silicone gasket. Because of these unique features, single-use bags manufactured with SterilEnz-II/G connectors provide greater sterility assurance and ease of use.
Figure 1: ()
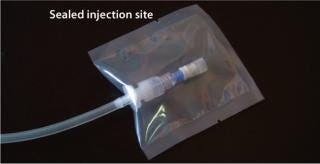
SterilEnz Samplers solve the common problem that occurs when using injection sites on single-use systems — that is, how does one maintain the sterility of the injection site itself in a nonsterile environment? The solution again is to seal the injection site inside a hermetic closure (Figure 2).
Figure 2: ()
Challenge Testing
The design and integrity of SterilEnz-II/G connectors has been verified through Liquid Tracer Immersion challenge testing using methylene blue dye. This is a common method for testing medical device packaging. SterilEnz test articles were irradiated and aged 4.5 years (real time). All test articles passed the test, thus verifying the design and integrity of the SterilEnz concept, including the often besmirched hose barb itself.
100% In-Process Integrity Testing
All SterilEnz-II/G connectors undergo nondestructive leak testing during manufacture. All parts are tested with a computer controlled integrity test using highly sensitive pressure transducers. The automated test method is highly accurate and reproducible. Based on the Liquid Tracer Immersion challenge test and the 100% in-process integrity test, SterilEnz fittings can be considered a truly hermetic closure.
Conclusion
SterilEnz-II/G sanitary flange connectors are a responsible choice when designing bags intended to use this style of connector. The hermetic closure protects the premounted gasket — this natural pairing solves a multitude of cleaning, reuse, and validation issues — as well as handling issues (e.g., dropped gaskets). At the same time, manufacturers of single-use products report that SterilEnz-II/G fittings are easier to assemble and offer overall lower cost to the bag design (parts and labor considered). Similarly, SterilEnz Samplers provide all the benefits discussed previously but as they apply to fluid sampling applications. Together, each of these products offers innovative improvements, cost reduction, and more options to end users.
REFERENCES
1.) 2004.ASTM F 1929: Standard Test Methods for Detecting Seal Leaks in Porous Medical Packaging by Dye Penetration, ASTM International, West Conshohocken.
2.) Denyer, S. 2006.Guide to Microbiological Control in Pharmaceuticals and Medical Devices, CRC Press, Boca Raton.
3.) McMaster, RC. 1982.Nondestructive Testing Handbook, American Society for Nondestructive Testing, Columbus.