Anion-exchange (AEX) products are commonly used as a polish step in product flow-through (FT) mode to bind impurities. Comparing resins with membranes and membranes with membranes is challenging because of the complicated and unique scaled-down model formats of the products offered by different vendors. A novel approach to AEX FT using a short, 5-cm length, packed-bed format with a faster operating flow rate is explained. The performance of commonly used AEX resins and membrane adsorbers, detailing dynamic binding capacity performance, efficiency, and a new prepacked column option for chromatography are compared. The data presented show that a high-performance AEX resin, when combined with the convenience of a GoPure™ prepacked column, competes well with the performance of membranes. It provides similar processing times and the added benefits of ease of packing at different scales in various column formats, with the ability to implement initial process design from early phase manufacturing to commercial manufacturing, reducing time to market.
Materials and Methods
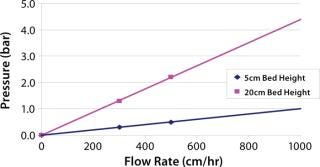
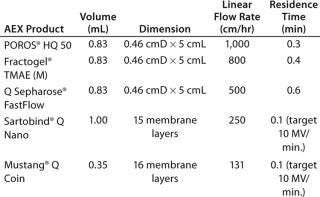
The scale-down model approach is summarized in Table 1. We evaluated five commonly used AEX products: three resins and two membrane adsorbers. The resin flow rate is the maximum flow the resin manufacturer stated that the resin could be flowed per operating instructions. The membrane flow rate was based on industry customers’ commonly used design space of 10 membrane volumes (MV)/minute. Although POROS® chromatography resins can be run at 2,000 cm/hr or faster, 1,000 cm/hr was evaluated as the upper limit for this evaluation. A 5-cmL POROS® column can be operated at 1,000 cm/hr with a pressure drop that allows for use with conventional low-pressure chromatography columns and systems (Figure 1).
Figure 1: ()
DNA Dynamic Binding Capacity: The AEX medium was precharged with 20 mM sodium phosphate, 1 M NaCl, pH 7.0, followed by an equilibration with 20 mM sodium phosphate, 50 mM NaCl, pH 7.0 (7.8 mS/cm). Each column/membrane was loaded with 2 mg/mL herring sperm DNA (Sigma D3159) in equilibration buffer (titrated to pH 7.0 with 0.2 M sodium phosphate dibasic, anhydrous) with a final conductivity of 8.8 mS/cm per the flow rates listed in Table 1. Binding capacities at 5% (C5) and 50% (C50) breakthrough were determined based on ultraviolet (UV) absorbance.
BSA Dynamic Binding Capacity: The AEX medium was precharged with 20 mM Tris, 1 M NaCl, pH 8.0, followed by an equilibration with 20 mM Tris, 1 M NaCl, pH 8.0 (1.1 mS/cm). Each column/membrane was loaded with 10 mg/mL bovine serum albumin (Sigma A7906, pI 4.7-5.3, MW 66 kDa) in equilibration buffer with a final conductivity of
Table 1: Scale-down approach for AEX chromatography resin and membrane adsorber comparison
Table 1: Scale-down approach for AEX chromatography resin and membrane adsorber comparison ()
POROS® HQ 50 Viral Clearance: Polyclonal human IgG (Sigma G4386, MW:155–160 kDa; pI: ∼6.9) was used for the model process. The salt concentrations evaluated and the corresponding conductivity values are summarized in Table 3. The column format was 0.46 cmD × 5 cmL, 0.83 mL. Operations were conducted at 1,000 cm/hr at room temperature (0.3 min residence time). The column was loaded with 5 mg/mL IgG with a 5% xenotropic murine leukemia virus (xMuLV, retrovirus) or murine minute virus (MMV, parvovirus) spike and samples taken to determine viral clearance.
Results and Discussion
POROS® HQ demonstrated good viral clearance in FT mode for the XMuLV model virus up to 150 mM NaCl (18 mS/cm) at pH 7, as summarized in Table 2. The MMV model virus showed good clearance up to 50 mM NaCl (8 mS/cm) in this new AEX FT format, suggesting that a shorter column run at a faster operational flow rate can achieve good viral clearance.
Table 2: Viral clearance on POROS® HQ 50 (1,000 cm/hr, 0.3 min residence time, 5 cm bed)
Table 2: Viral clearance on POROS® HQ 50 (1,000 cm/hr, 0.3 min residence time, 5 cm bed) ()
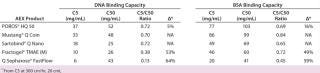
The DNA and BSA binding capacity data are summarized in Table 3. POROS® HQ 50 demonstrated the highest DNA binding capacities at 5% breakthrough of all five AEX products assessed. The C5/C50 ratio is a measure of the mass transfer capability of the media and a way to characterize the efficiency of binding. As the C5/C50 ratio approaches 1.0, the resin/membrane becomes more efficient. The more efficient the mass transfer, the less effect flow rate has on capacity. The POROS® HQ C5/C50 ratio is as efficient as the membranes and significantly more efficient than Fractogel® TMAE and Q Sepharose® Fast Flow for DNA binding. The capacity at 5% breakthrough on the 5-cmL column at 1,000 cm/hr was compared with the capacity on a 20-cmL column run at 300 cm/hr. POROS® HQ had minimal chang
e in performance in the two formats compared to the other two resins. In addition, with POROS HQ, the DNA capacity is high under a wide range of operating conditions (30–35 mg/mL at pH 6.0–9.5 with 150 mM NaCl and >20 mg/mL at pH 7.0 with up to 400 mM NaCl, data not shown). POROS® HQ ranked second highest for BSA C5 dynamic binding capacity and shows similar capacity and mass transfer efficiency to the membranes.
Table 3: DNA and BSA dynamic binding capacity summary of AEX comparison study in order of DNA capacity at 5% breakthrough
Table 3: DNA and BSA dynamic binding capacity summary of AEX comparison study in order of DNA capacity at 5% breakthrough ()
Conclusion
This novel approach to AEX flow-through polish chromatography as a short, disk-like column with faster operating flow rate delivers increased flexibility when designing a purification scheme. The properties of POROS® HQ 50 drive increased throughput and smaller column sizes, ultimately a column that can be sized based on capacity for impurities. This study has shown that POROS® HQ 50 competes well with the performance of membranes with the added benefits of ease of packing at different scales in various column formats and the ability to implement initial process design from early phase manufacturing to commercial manufacturing, reducing the overall process development costs and decreases time to market. In addition, POROS® HQ has been shown to have high impurity binding capacity and clearance over a range of process conditions, including high conductivity conditions (data not shown). This decreases the need for dilution of the feed stream or inclusion of a diafiltration step prior to column loading, helping to make the process more efficient and cost-effective.
For added convenience, Life Technologies now offers GoPure™ prepacked chromatography columns for maximum convenience, providing faster time to process and faster time between processes. This new approach to AEX FT polish chromatography increases process development flexibility as well as offers a more effective approach to this process step compared with improperly, oversized soft-gel columns. In addition, ease of packing at different scales in various column formats and the ability to implement initial process design from early phase manufacturing to commercial manufacturing helps reduce overall process development costs and decrease time to market.
About the Author
Author Details
S. Parra, E. Haimes, R. Garretson, and C. Gebski are with Life Technologies, 35 Wiggins Avenue, Bedford, MA 01730; www.lifetechnologies.com.©2012 Life Technologies Corporation. All rights reserved. The trademarks mentioned herein are the property of Life Technologies Corporation or their respective owners. Mustang is a registered trademark of Pall Corp. Sartobind is a registered trademark of Sartorius AG. Fractogel is a registered trademark of Merck KGAA. Sepharose is a registered trademark of Pharmacia Fine Chemicals Inc. All other trademarks are the sole property of their respective owners.