Figure 1: Sartopore® Platinum TwinPleat ()
Sterile filtration using 0.2-µm rated filters is a critical step in upstream and downstream biomanufacturing alike. Typical applications comprise sterile media addition into the bioreactors, cell harvest clarification post-depth filter, chromatography column protection, and final sterile filtration of purified bulk drug substance. Total throughput, flow rate, unspecific adsorption, and wettability of sterile filters can have direct impact on total cost of ownership.
There have been innovative application-specific approaches in sterile filtration such as the Sartopore®2 XLG 0.8/0.2 and Sartopore®2 XLI 0.35/0.2 PES filters from Sartorius Stedim Biotech (SSB). Both filters have different prefilter membrane retention ratings designed for optimum performance with fluids having specific particle-size distribution characteristics. Sartopore®2 XLG shows higher capacities for serum-free, hydrolysate supplemented media and cell culture harvests, whereas Sartopore®2 XLI performs the best on CD media and viscous solutions. These filters have reduced total filtration area by >50% in some large-scale applications while eliminating the need for prefilters.
SSB is introducing its third generation PES 0.2-µm filter: Sartopore® Platinum. It is in a class by itself by providing outstanding total throughput and flow rate due to its high EFA (1 m2/10″), resulting in one of the lowest filtration cost per liter. Its patent-pending TwinPleat® technology (photo), with alternating sequence of long and short pleats positioned at an angle to cartridge core, improves hydrodynamics with entire membrane easily accessible during filtration.
Figure 1: Sartopore® Platinum TwinPleat ()
Sartopore® Platinum requires minimal WFI wetting volumes with
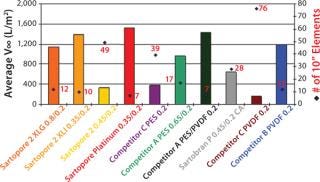
This revolutionary filter was tested with various media, harvest, and downstream feeds showing positive results. One example of its relative performance for fully chemically defined media with high lipid content is shown here. Sartopore® Platinum, XLG & Comp. B PES/PVDF 0.2 were the lead candidates with V∞ ∼ 1,530, 1,395, and 1,435 L/m2 respectively. When this high throughput/m2 is coupled with 1 m2 fully accessible area, only 7-10″ elements would be required to filter a 5,000 L batch in two hours with Sartopore® Platinum (Figure 2). With other high-density crescent pleat constructions, scalability issues have been reported.
Figure 1: ()
Figure 2: ()
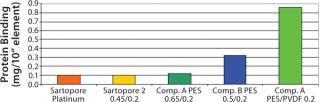
Sartopore® Platinum represents a new class of sterile filtration offering exceptional total throughput performance with minimal protein binding, easy wettability prior to Integrity Testing, and dry and reverse steambility for cartridges. The overall cost of ownership for filtration is reduced for many challenging applications due to its unique nature.
About the Author
Author Details
Mandar Dixit is director of field marketing, filtration technologies, North America, 1-800-368-7178 x8520; [email protected].