Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
August 1, 2013

Analytical techniques that measure protein quantity and quality are used in nearly all stages of research, process development and manufacturing of biotherapeutics. Biopharmaceutical companies have enthusiastically adopted Pall ForteBio’s Octet systems due to their broad utility in protein quantitation and functional characterization combined with no need for labels, real-time monitoring, enhanced throughput, decreased sample preparation requirements, and low cost of operation.
Concentration Measurement
The Octet platform uses ForteBio’s proprietary Biolayer Interferometry (BLI) technology to measure concentration in 96-well and 384-well microplate formats. Biosensors coated with a capture molecule (called the ligand) are dipped into analyte solutions to measure binding interactions. A standard curve is generated using known amounts of the protein analyte, and unknown sample concentrations are interpolated from the standard curve.
Quantitation Applications in Drug Development Using Octet Systems
Research and Early Process Development — Early Clone Selection: Thousands of hybridoma or phage clone samples are screened to determine positive binders and protein secretion levels. Titer measurements are used to normalize the functional activity of these clones. Quantitation measurements also are used to determine loading levels for chromatography columns during small-scale purification.
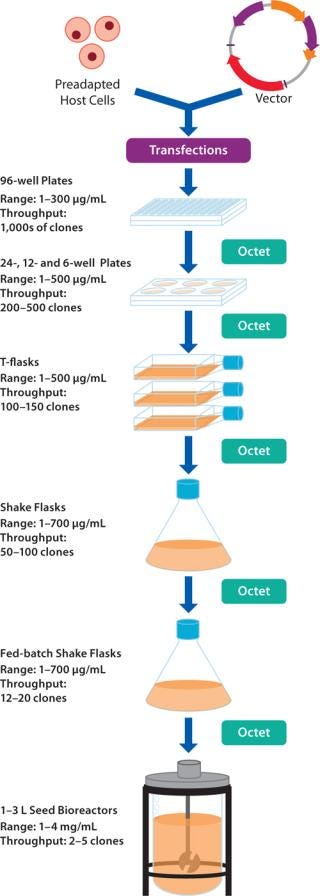
Cell Line Development: Harvest samples are screened to select high-expressing clones and perform growth condition optimization during scale-up procedures involving 96-, 24-, and 6-well plates, fed-batch shake flasks, and bioreactors (Figure 2) (1). Data acquisition and subsequent data analysis can be performed rapidly for hundreds of samples, bypassing traditional processing bottlenecks.
Octet Platform Benefits: Upstream Bioprocess
Antibody and protein concentrations can be determined in crude matrices such as cell lysates or hybridoma supernatants.
Assay dynamic range is greater than two orders of magnitude, enabling a single quantitation assay to be used throughout cell-line development.
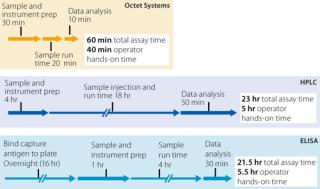
Rapid quantitation can be performed with minimal user involvement. Analysis of 70 samples on an Octet RED96 system requires only 55 minutes including operator hands-on time, whereas ELISA or HPLC assays require at least 22 hours, including several hours of analyst involvement (Figure 1).
Samples are fully recoverable, which is advantageous when working with low volumes and precious samples.
Octet systems are easy to learn and operate, allowing rapid integration into laboratory workflows.
Downstream Process Development: Efficient development and understanding of purification processes for antibodies and recombinant proteins is a critical need for companies due to increasingly stringent regulatory requirements. The Octet platform can be used to quickly determine the impact of multiple process variables at different stages of the purification process, and to identify optimal conditions that provide protein product with the desired yield, binding specificity and potency (Figure 3).

Dynamic Binding Capacity (DBC) of Chromatography Columns: Affinity chromatography often is the first purification procedure performed on harvested cell culture samples. Quick determination of DBC using high-performance liquid chromatography (HPLC) or A280 spectroscopy is hampered by the presence of large amounts of host-cell proteins in flow-through fractions. Specific detection of the protein of interest among contaminants is straightforward with Octet systems (4).
Binding, Wash and Elution Conditions: Numerous chromatography binding and elution conditions are tested during optimization studies, including different buffer compositions, salt, pH, and operating temperatures. High-throughput tools such as 96-well filter plates are used to screen these process variables. The impact of different conditions on product titer and quality, and contaminant levels can be analyzed rapidly on Octet systems.
Contaminant Testing: Assays for residual protein A and HCP can be fully automated on Octet 384 systems. The Octet assay for leached protein A is highly sensitive with a lower limit of quantitation (LLOQ) of 0.20 ppm, has >2.5 logs of dynamic range, and can be completed in an hour and 45 minutes for each 96-well plate, with minimal analyst involvement (4). The HCP assay on the Octet 384 system reduces analyst hands-on time to 30 minutes from ~2.5–3.0 hours with ELISA for one to three assay plates (3, 5).
Octet Platform Benefits: Downstream Bioprocess
Octet assays are significantly faster to run than ELISA and HPLC assays.
Octet assays can be automated with liquid handling systems for walk-away screening.
Quality Control (QC): Octet systems provide robust and highly reproducible assays for protein concentration and functional activity, and are suitable for operation in QC and manufacturing environments. These assays are often used to support drug potency, lot-to-lot variability, and stability studies.
Activity assays are critical because they differentiate active protein from inactive or clipped variants because those species will not bind the ligand. Active protein concentration can be determined using a binding assay on the Octet platform by immobilizing a specific ligand against the target analyte onto the biosensor, then measuring its binding interaction with the analyte (Figure 4) (6).
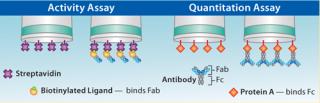
Fc-Receptor Assays: Fc-Receptor (FcRn, FcγRI, FcγRIIa, FcγRIIb, FcγRIIIa) binding assays are used to determine whether various stresses or process conditions result in changes in an antibody’s Fc region. The assay uses an Fc-receptor immobilized on a streptavidin or amine-reactive biosensor, which selectively detects binding of antibodies or Fc-fusion proteins.
Conclusion
Titer and functional activity assays on Octet systems are useful for a broad array of applications in target identification, lead selection, process development, quality control, and manufacturing. In early stages of drug development, Octet systems provide the high throughput needed to screen through large libraries of candidate drug molecules. In later stages of process development and QC, Octet systems provide the required reliability, robustness and accuracy in measurement. The extensive utility of this single platform makes the Octet instrument unique in its ability to deliver high value across a wide range of application needs in biopharmaceutical discovery, development and manufacturing processes.
Octet Platform Benefits: Quality Control
Octet systems are designed for GLP/GMP environments and provide 21 CFR Part 11 compliance tools.
Activity assays reveal subtle differences in binding activity between production lots.
Quantitation assays provide a direct measure of the biological activity of analyte(s) (Figure 4).
Assays can be easily transferred to manufacturing operations.
About the Author
Author Details
Rashi Takkar is an application scientist at Pall ForteBio, 1360 Willow Road, Menlo Park CA 94025; 1-650-722-4844; [email protected]; www.fortebio.com.
1.) Application Note 13 2013. Rapid, Reliable Quantitation of Fc-Fusion Protein in Cell Culture Supernatants, ForteBio, Menlo Park.
2.) Schuessler, D 2011. Fully Automated CHO Host Cell Protein Quantitation in Monoclonal Antibody Therapeutics, ForteBio, Menlo Park.
3.) Tech Note 18 2010. Dip and Read Residual Protein A Detection Kit, ForteBio, Menlo Park.
4.) Do, T 2008. A Rapid Method for Determining Dynamic Binding Capacity of Resins for the Purification of Proteins. Prot. Expr. Purif. 60:147-150.
5.) Application Note 11 2013. Enhancing Efficiency and Economics in Process Development and Manufacturing of Biotherapeutics, ForteBio, Menlo Park.
6.) Application Note 12 2013. Validated Quantitation and Activity Assay of Antibody Fragment Molecule (Fab) for Process Development and Quality Control, ForteBio, Menlo Park.
You May Also Like