Antibody production platform processes have been widely adopted in biomanufacturing, but many unit operations are not suitable for integration and automation. Here we describe the work of integrating unit operations by transforming a column operation to a more robust cassette format. We have selected a biomolecule-friendly buffer (phosphate) to eliminate, or delay, the performance of a circulating tangential flow ultrafiltration/diafiltration (UF/DF) operation, so the harvest-to-purified-bulk process can be integrated, resulting in a single, direct-flow operation, that reduces the batch process time from about two weeks to six hours.
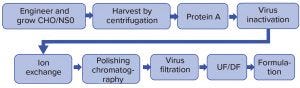
Figure 1: Antibody production platform
Antibody production platform processes were established in the late 1990s. In the past 20 years, biomanufacturers have been using engineered Chinese hamster ovary (CHO) and NS0 cells to produce antibodies, bispecific biomolecules, and other fusion proteins. Figure 1 shows the current platform process.
Such processes are associated with the following problems:
They are labor intensive because they typically comprise many unit operations from harvest to formulation.
They are inefficient because each batch requires about two weeks to process from harvest to formulated bulk drug substance.
Chromatography unit operations are not robust because a simple introduction of air to a column can compromise the integrity of the packed resin bed and risk loss of a multimillion-dollar batch.
To overcome these challenges, we conducted studies to integrate and consolidate the current platform process from many independent unit operations to one. Doing so significantly reduced the processing time and enables future automated continuous processing without operators on the manufacturing floors.

Table 1: MabSelect SuRe LX protein A process steps and buffers
Material and Methods
Materials: The antibody starting material is an IgG1 produced by a Chinese hamster ovary (CHO) cell culture fed-batch process. Affinity chromatography is operated using MabSelect SuRe LX resin from GE Healthcare and operated using a peristaltic pump or ÄKTA chromatography workstation, also from GE Healthcare. Buffers were prepared in OED CMC Biologics Bioprocessing group at AbbVie. The depth filters were from Millipore, the Mustang Q anionexchange membrane filter was from Pall, the Virosart Max 0.1 μm prefilter was from Sartorius Stedim Biotech, and the Viresolve Pro virus filter was from Millipore. JSR Life Sciences and SPF Technologies provided the Chromassette chromatography platform.

Table 2: Buffer reduction from 15 to three stock solutions
Methods: Size-exclusion chromatography (SEC) high-performance liquid chromatography (HPLC) was used to assess the level of aggregates, and the NanoDrop2000 system from Thermo Fisher Scientific was used to assay IgG concentration by absorbance at 280 nm. The QuantiChrom phosphate assay kit (DIPI-500) from BioAssay Systems was used to assay phosphate concentration, and an enzyme-linked immunosorbent assay (ELISA) to assay host-cell protein (HCP) content.
Results
Buffer Simplification: To integrate the process, the buffers used in the platform had to simplified and reduced. Our current platform uses ~15 buffers consisting of Tris, acetate, and phosphate buffers as well as NaOH and HCl. The following experiments demonstrated that Tris and acetate buffers can be eliminated.
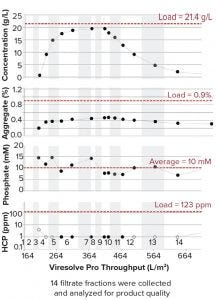
Figure 2: Product profile of flowthrough purification before ultrafiltration and diafiltration
Table 1 shows that the protein A chromatography methods and the transition from Tris and acetate buffers to the phosphate buffer scheme. The typical platform elution was ~2 CV at 20–30 g/L. 10 mM phosphoric elution buffer eluted a broader peak leading to larger eluate volume. If the equilibration wash is lowered to pH 6.0, the elution volume can be reduced to ~2.5 CV using the 10 mM phosphoric acid (H3PO4) eluent.
The protein A eluate pool eluted using 10 mM H3PO4 was titrated to pH 3.5 using 0.2 N hydrochloric acid (HCl) and held for 30 minutes for viral inactivation (VI), then titrated to pH 6.8 using 0.1 N sodium hydroxide (NaOH). The neutralized pool was then processed through the remainder of the purification process (depth filter + Q anion-exchange filter + virus filtration) using 10 mM phosphate buffer at pH 6.8 as a single direct-flow process step. The flow through was collected in 14 fractions.
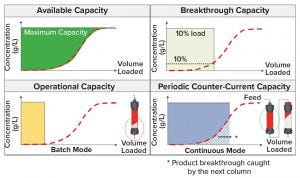
Figure 3: Increase use of protein A capacity
Results in Figure 2 show that the middle fractions reached concentrations of 19-20 mg/mL. Aggregates and HCP were very low. More important, the mean phosphate concentration was 10 mM. Therefore, if the formulation is in 10 mM phosphate at pH 6.8, the final UF/DF unit operations can be eliminated, and the total number of buffers can decrease by half or even more with three stock solutions of sodium chloride (NaCl), NaOH, and H3PO4 (Table 2).
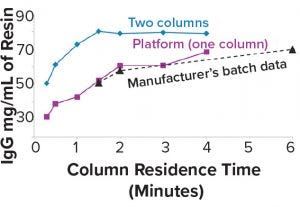
Figure 4: Higher loading capacity with two columns
Multicolumn Protein A Chromatography: To increase the final purified bulk concentration, without the UF/DF step at the end of the process, it is necessary to increase protein concentration in protein A eluate pool. This can be achieved by loading the protein A column to a much higher mass capacity. Figure 3 shows that loading too much protein will have significant product breakthrough and reduce the step yield. That problem can be prevented by using two columns in series.
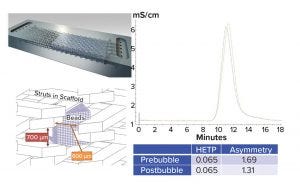
Figure 5: Scaffold support and HETP tests (HETP = height equivalent to the theoretical plate)
Figure 4 shows that the first column can be loaded to >80 g/L on MabSelect Sure LX resin at a 1.5-min residence time. This can reduce resin usage by 38% and increase the protein A eluate concentration. Newer protein A resins, such as the PrismA resin from GE Healthcare and Amsphere A3 resin from JSR Life Sciences, can further increase protein A eluate concentration.
Column to Cassette Transformation: To overcome process robustness issue of a column operation, the new Chromassette platform was introduced to replace the column. This platform has an integrated scaffold support every ~700 μm that prevents bead compression even at high flow rates and restricts the movement of packed resin bed. In this way, air cannot break up a packed bed and create channels. Figure 5 shows that air intentionally introduced into a Chromassette platform packed with MabSelect SuRe LX resin had virtually no impact on the height equivalent to the theoretical plate (HETP) and peak asymmetry results.
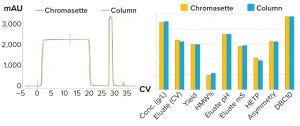
Figure 6: Comparing protein A column with Chromassette unit shows nearly identical results.
Figure 6 shows test results comparing protein A chromatography performances using a traditional column and the Chromassette system. The chromatograms and process performances are nearly identical.
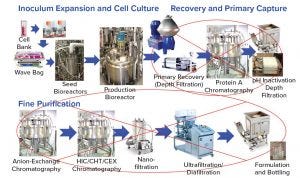
Figure 7: Platform process can be simplified
Integrated Process Concept: With the introduction of the Chromassette platform to replace columns and the development of a protein A membrane, the current platform process from harvest to purified bulk can be converted to a cassette format. A new concept is proposed that eight independent unit operations in Figure 7 are integrated into a one-unit operation as shown in Figure 8. This results in a uniform integrated recovery and purification (IRP) system. The IRP platform flows in series through the process train starting with harvest depth filters followed by a protein A Chromassette system, a virus inactivation chamber, postvirus inactivation depth filters, Q filter, a polishing Chromassette unit, and finally, the virus filter. The purified bulk can then be filtered through a 0.2 μm filter into a bag for storage.
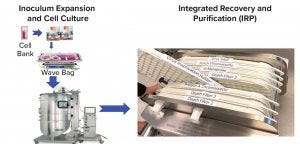
Figure 8: Concept of integrated bioprocess
Figures 9 and 10 show a proof of concept of the IRP platform. In Figure 9, an ambr250 bioreactor (from Sartorius Stedim Biotech) harvest of 250 mL was processed through two depth filters, 0.2-m filter, and a protein A Chromassette unit. After virus inactivation, material was further processed through a depth filter, 0.2‑μm Q filter, 0.1-μm filter, 20-nm virus filter, and finally another 0.2-μm filter into a bag of purified bulk drug substance.
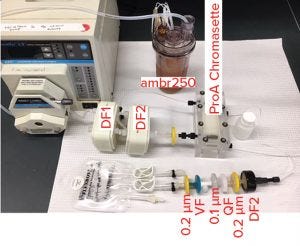
Figure 9: Proof of concept for integrated
bioprocess
Figure 10 shows chromatograms of the harvest and protein A as well as the subsequent purification steps following virus inactivation. The gradual increase observed in the UV trace is not a result of product breakthrough, but rather a result of buffer dilution from depth filters. The purified bulk produced was 20 mg/mL concentration at pH 5.9 and contained 0.6% aggregate. More important, the entire IRP process was performed in six hours instead of the two weeks needed to complete the current manufacturing platform process.
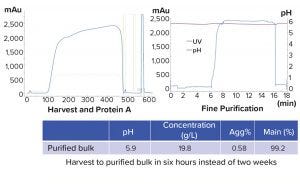
Figure 10: Results of integrated bioprocess (Agg% = percent of aggregates)
Future Bioprocessing Without Operators
Because the IRP platform is using robust filters and the Chromassette platform, and replacing disposable filter units is simple, robots could be programmed for future bioprocesses (Figure 11). Rather than on the manufacturing floor, operators could be in control rooms monitoring operation. The paradigm shift reduces the chance of contamination and operator errors introduced by human operators. In addition, because an IRP platform is compact, it can be housed in a small space, and the environment can be kept clean to prevent contamination. That could reduce the manufacturing footprint by at least 80% and shorten the time and expense for facility construction. Ultimately, such new systems have the potential to reduce manufacturing costs and ensure product safety.
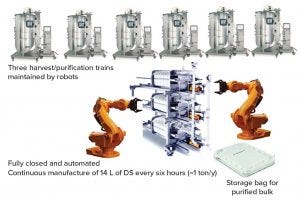
Figure 11: Future bioprocess without operators
Advantages of an Integrated Bioprocess
The number of monoclonal antibody platform purification process buffers can be reduced significantly by using only phosphate buffers. With the introduction of the Chromassette platform to replace column chromatography, the unit operation is more robust. In addition, after transitioning a column into cassette format, the entire process from harvest to purified bulk can be integrated into one unit operation and completed in six hours. In the future, such integrated bioprocesses may be operated continuously at large scale by robots, producing approximately one ton of antibody per year within a small manufacturing footprint and with few required equipment systems.
Acknowledgments
Many scientists in Bioprocess Development and Analytical Development from the AbbVie OED CMC Biologics teams contributed to this article. We acknowledge Richard Wu for performing a number of downstream experiments and Dan Rush for many analytical assays. Thanks to Barry Wolf and Nikki Nguyen and others in BioProcess group for producing the cell culture harvest.
Corresponding author Ping Y. Huang is the director and head of bioprocess development. Yekaterina Lin is a senior scientist of bioprocess purification development. Robert J. Duffy is a principal scientist of bioprocess purification development. Amit Varma is senior director and head of CMC biologics, at CMC Biologics, Oncology Early Development, at AbbVie; [email protected]. This article is adapted from the authors’ poster at the 2019 BPI West conference.




















