Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
The biopharmaceutical industry generally acknowledges that upstream and downstream aspects of drug-substance manufacturing are experiencing a capacity mismatch. Today, many recombinant proteins can be produced at expression titers of 3 g/L, with some yields exceeding 10 g/L. Such titers represent 100-fold increases in production capability compared with values from twenty years ago (1, 2). Increases in cell-culture density and improvements to perfusion-mode bioreactor systems hold promise for increasing yields further still. Such developments, combined with the broad availability of concentrated fed-batch options for both pilot- and commercial-scale production, now require downstream teams to process harvests ranging from 5 kg to 40 kg per batch (1). Despite improvements to chromatographic media capacity and membrane performance, however, downstream operations have not experienced corresponding gains in productivity. And whereas process intensification efforts are helping to reduce facility requirements for upstream operations, downstream footprints generally have increased to accommodate the sheer volume of material that must be processed.
Biomanufacturers continue to explore continuous downstream processing (CDSP) as a scalability strategy. Distributing purification and/or filtration steps over time and equipment could enable downstream process engineers to handle more material and in a smaller footprint than would be possible in a conventional, large-scale batch process. CDSP also holds promise for reducing the complexity and risks to product quality that are associated with significant scale-up of purification steps.
Several approaches are possible (3). In fully continuous mode, all unit operations run continuously, and connections flow across units. In end-to-end mode, inputs and outputs are continuous, but intermediate unit operations can be conducted in batch mode. Companies also can pursue a hybrid strategy to align their operational needs and technical capabilities.
BPI most recently explored the state of CDSP in a 2019 roundtable discussion (1). At the time, participants indicated that commercial-scale processes largely remained in developmental stages. Among the contributors was Margit Holzer (scientific director at Ulysse Consult and BPI’s newest editorial advisor). I corresponded with her early in 2022 to learn about recent advances in CDSP technologies, technical requirements for transitioning from batch to continuous operations, and ongoing needs for commercial-scale implementation.
Holzer is a recognized expert in downstream processing of biopharmaceutical products. She holds a PhD in biotechnology from the University of Natural Resources and Applied Life Sciences in Austria, and her industry experience spans over 25 years, including leadership roles at Boehringer Ingelheim and Novasep. She currently serves as an industry consultant and BPI Academy instructor.
Our Conversation
Aside from being able to process more material, what benefits can be reaped by transitioning from batch mode to CDSP? A major benefit is improved product quality. CDSP reduces product holding and processing times while enhancing process consistency and product purity.
Operationally, CDSP enables large amounts of product to be prepared using the same types of equipment and facilities — without necessitating major investments or process transfers that can prolong project timelines significantly.
Continuous operations also can reduce capital expenditure (CapEx) and production costs. Such economic impacts are important considerations, especially for products that must be processed in large volumes both up- and downstream.
In 2019, you contributed to a roundtable discussion in which participants suggested that commercial-scale CDSP remained in its infancy (1). Is that still the case? More technologies than ever before are being developed for industrial scales, facilitating continuous operations. But I agree that implementation of CDSP typically occurs step by step rather than as a radical change of operations. For instance, technologies for continuous harvesting operations (e.g., centrifugation and tangential membrane filtration) already have been implemented at manufacturing scales.
Technologies that enable adjustments to compositions of solutions are facilitating continuous operations and making positive impacts on facility space and production costs. Examples are in-line dilution (ILD) or conditioning (ILC) systems for buffers. Such technologies adjust the pH and/or conductivity of process solutions. The industry also uses tubular heat exchangers to adjust temperatures during continuous processes.
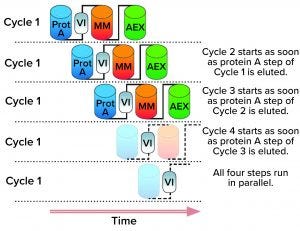
Figure 1: Illustration of a multistep multicolumn chromatography (MMC) process depicting how an upstream load is treated over several cycles of protein A (Prot A) capture, viral inactivation (VI), mixed-mode chromatography (MM), and anion-exchange chromatography (AEX) (4). (IMAGE COURTESY OF NOVASEP)
Companies have started to implement mutlicolumn chromatography (MCC) systems for one or two process steps with the goal of reaching CDSP after gaining good manufacturing practice (GMP) production experience (Figure 1).
Preparative chromatography steps have been difficult to integrate within continuous operations. Why? In a batch process, downstream scientists often need to collect fraction analyses (obtained by off-line analytics) before they can pool a process solution and load the next downstream step. For continuous operations, we need on-/in-line analytics that enable timely control of a separation process.
How might MCC facilitate transitions to continuous operations? In what cases would drug manufacturers consider its implementation? Based on a robust control strategy, we already can develop a MCC process by running two or more chromatography columns sequentially or in parallel (Figure 1).
MCC might be implemented if buffer and chromatographic media consumption also need to be reduced. That can be achieved by MCC applying the same materials as in batch mode, although a specific process characterization program would need to be executed. Thus, the number of columns, their bed heights, product loading and buffer sequences, and column connections all will need to be defined.
MCC also can be applied to improve separation efficiency (and increase the purity of a targeted molecule) by increasing column efficiency in the separation zone, collecting pure product, and recycling side fractions. Such processes are applied for polishing steps. Again, there will be a need for specific process characterization and development work, often combined with process simulation.
GMP-compliant equipment and software are available for implementation of all the MCC processes that I described above. Currently, products derived from such installations — including some monoclonal antibodies (MAbs) and vaccines — are being used for clinical trials and initial commercial applications.
I’ve encountered less discussion about filtration than about chromatography in recent literature on CDSP. Is filtration amenable to continuous operation? Often, tangential-flow filtration (TFF) steps are applied during harvest and drug-product formulation and concentration. Redeveloped TFF modules such as ATF (alternating tangential flow) technologies from Repligen enable continuous-flow operations.
Volumes of final product are often small, and drug-substance production and processing typically are decoupled from drug-product preparation and formulation. Thus, filtration steps are natural places to define batch sizes and to collect and homogenize the material coming from CDSP.
New technologies such as single-pass TFF concentration or diafiltration — often derived from equipment used in the food industry — are being developed to achieve continuous operation. Flow velocities, membrane area, and pressure control are important design parameters for single-pass processes.
How does the decision to implement continuous processing affect membrane selection? Are certain materials of construction more amenable to continuous operation than others? Membrane modules must be designed specifically for single-pass concentration and for single-pass diafiltration or ATF steps. It is not a question of membrane material, but of module and process design. Clearly, flow rates and pressure resistance need to match membrane characteristics.
What considerations arise for bioburden control and viral safety? To control bioburden, particle-load direct-flow filters (such as depth, bioburden-reducing or sterile filters) need to be integrated into a CDSP process line. Filter position (including for buffer exchanges and upstream production steps) should be defined for a control strategy based on batch-processing experience and risk analyses. Alternating parallel filters are usually the easiest solution.
As with bioburden filtration, the easiest way to implement viral filtration in a CDSP is to implement a process design with alternating parallel filters.

Figure 2: The Cadence single-use viral inactivation system for batch and continuous processing (5). (IMAGE COURTESY OF PALL BIOTECH)
Which viral safety operations are most difficult to adapt to continuous processing? Low-pH treatment for viral inactivation can be challenging because very precise residence times and pH transitions are necessary. But I believe that such technological hurdles have been negotiated, with many emerging viral-inactivation systems being designed for batch and/or continuous processes (Figure 2).
MCC separations are perhaps the most necessary element to study for ensuring viral clearance in CDSP. Risks for viral contamination during continuous operation also need to be assessed and mitigated.
What must happen for biomanufacturers to realize fully continuous operations at production scales? Fully end-to-end CDSP has made major technological progress during the past few years. New technologies that enable the integration of unit operations into a connected and controlled process line are key for CDSP.
Connection of unit operations requires alignment of flow rates and control of residence-time distribution. Product composition must be adapted for subsequent processing units. And instrumentation must provide for all the necessary controls. That is true not only for product processing, but also for necessary rinsing or resin-regeneration steps. Quality data must be collected and processed on/in line to enable product release.
Could you say a bit more about industry needs for real-time release testing (RTRT) of product? That topic is important now, especially for products that need short release cycles (e.g., some cell therapies). The same is true for continuous and intensified processes. Intermediates and potentially drug substances need to be analyzed and released as they are processed.
One strategy for accomplishing that is to develop on-line analytical methods based on spectroscopic methods using different wavelengths — e.g., ultraviolet and ultraviolet–visible (UV, UV-Vis), infrared and near infrared (IR, NIR), and nuclear magnetic resonance (NMR) spectroscopies — for detecting target products or impurities in process streams.
Another option is to use rapid off-line methods for detection and quantification of endotoxins, bioburden, viruses, mycoplasma species, and other such contaminants. Product-related substances and/or impurities could be analyzed with high-performance analytical methods such as ultraperformance liquid chromatography coupled with UV and/or mass spectrometry (UPLC-UV/MS). Sampling positions and frequency can be determined based on risk analyses.
Tools for data tracking, analysis, and control are important in this context. They can be connected directly to a process line either to communicate alerts to operators or to stop operations based on out-of-specification (OOS) situations.
Testing time still needs to be reduced from weeks or days to hours for some release methods: e.g., compendial virus testing to release cell-culture broths, cell-culture–based potency tests used to release final product, and sterility testing.
References
1 Montgomery A, et al. Making Downstream Processing Continuous and Robust. BioProcess Int. 17(6) 2019: 38–48; https://bioprocessintl.com/manufacturing/continuous-bioprocessing/making-downstream-processing-continuous-and-robust-a-virtual-roundtable.
2 Rader R. Top Trends in Biomanufacturing. BioProcess Int. 19(1–2) 2021: 26–29; https://bioprocessintl.com/business/economics/top-trends-in-biopharmaceutical-manufacturing-2020.
3 Holzer M. Is Continuous Downstream Processing Becoming a Reality? BioProcess Int. 15(5i) 2017: https://bioprocessintl.com/manufacturing/continuous-bioprocessing/continuous-downstream-processing-becoming-reality.
4 Flouquet M. Process Intensification and Continuous Bioprocessing. Novasep: Pompey, France, 2018; https://www.novasep.com/landing-page/eq-biopharma-intensification-continuous-bioprocessing-white-paper.html.
5 Data Sheet: Cadence Virus Inactivation System. Pall Corporation: Port Washington, NY, 2020.
Brian Gazaille is associate editor of BioProcess International, part of Informa Connect, PO Box 70, Dexter, OR 97431; [email protected]; 1-212-600-3594.
You May Also Like