Fluorescent Nanosensors: Real-Time Biochemical Measurement for Cell and Gene Therapies
May 28, 2020
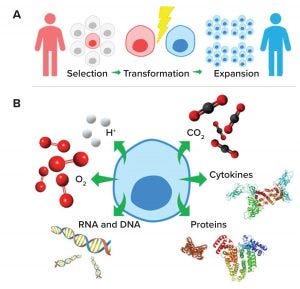
Figure 1: (a) Cell and gene therapy development pathway from patient/donor (red) to recipient (blue); (b) subcellular molecules and ions important for effective cell and gene therapy cultures
Cell and gene therapies are destined to transform the methods by which global healthcare challenges are approached and overcome (1). The US Food and Drug Administration is reviewing and approving an increasing number of cell and gene therapy products (2), and biopharmaceutical developers are dedicating immense resources to realizing the enormous potential of these therapeutics. Therefore, technologies that facilitate their effective and efficient manufacture will accelerate cell and gene therapies’ transition from medicines of the future to medicines of the present (3).
Cell therapies are administered as live cells from a patient (autologous) or donor (allogeneic) for therapeutic benefit (e.g., blood transfusions and stem-cell therapy). Gene therapies can be considered as the addition, silencing, correction, or reprogramming of genetic material through application of vectors for therapeutic benefit (e.g., Luxturna voretigene neparvovec-rzyl from Spark Therapeutics for inherited retinal RPE65 gene disease) (4).
Cell and gene therapies require extracting and selecting autologous or allogeneic cells, then transforming them to a final product that is therapeutically advantageous (Figure 1a). Transformed material is expanded ex vivo and readministered for therapeutic benefit. Chimeric antigen receptor (CAR) T-cell therapies are examples of cell and gene therapies. They are developed by reprograming immune cells to target cancer cells. The FDA has approved two CAR T-cell therapies: Kymriah (tisagenlecleucel) from Novartis and Yescarta (axicabtagene ciloleucel) from Kite Pharma for the treatment of B-cell acute lymphoblastic leukemia and relapsed or refractory large B-cell lymphoma, respectively (5, 6).
Bioreactors provide the growth conditions required to increase cells and vectors to quantities sufficient for therapeutic doses. The vessels provide a sterile environment for eukaryotic or microbial culture conditions (e.g., temperature, aerobic/anaerobic, and pH), with the addition of nutrients (e.g., carbohydrates, proteins, and lipids) and growth factors (e.g., cytokines), which typically are monitored extracellularly (7). Extracellular measurements provide an excellent indication of cell-culture inputs and outputs. However, they do not provide a direct indication of subcellular processes, which are where many biochemical changes important for effective cell culture occur (Figure 1b).
Therefore, the introduction of tools that permit real-time analytics of subcellular biochemical processes could play a significant role in the optimization of cell and gene therapy manufacturing and facilitate the transition of these products to mainstream therapeutics.
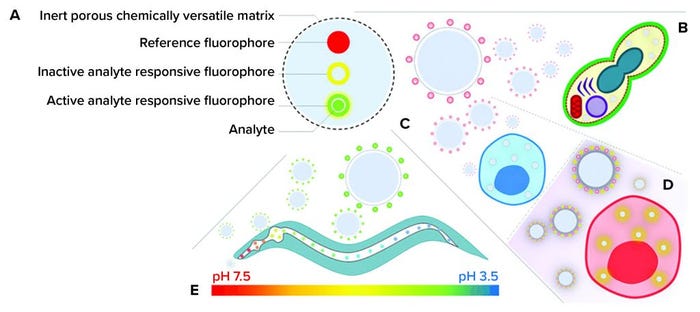
Figure 2: (a) Ratiometric fluorescent nanosensors; (b) delivery to Saccharomyces cerevisiae (26) and human mesenchymal stem cells (hMSCs) (27) for (c) determining intracellular processing of silica magnetic iron nanoparticles and (d) photo induced phenotypic changes (28) as well as (e) pharyngeal and intestinal pH mapping in Caenorhabditis elegans
Background
Fluorescent nanosensors are emerging technologies that permit silent measurement of key biochemical parameters to high spatial and temporal resolution (8). Nanosensors can be manufactured to a range of sizes, from 10 nm to 1,000 nm in diameter (9). They are manufactured from inert biocompatible materials (10) that bind to or encapsulate fluorophores (Figure 2a).
Fluorescent nanosensors typically consist of two or more different fluorophores that are reference and analyte-sensitive entities. A reference fluorophore produces a stable fluorescence emission upon excitation, which is insensitive to an analyte of interest. An analyte-sensitive fluorophore exhibits a dynamic fluorescence emission dependent on the amount of target. Multiple targets can be detected by incorporating more than one analyte-sensitive fluorophore (11). The combination of reference and analyte fluorophores enables accurate ratiometric measurements (12).
Fluorescence techniques have a greater sensitivity than do other spectroscopic methods. Therefore, nanosensors can be delivered at subharmful concentrations and accurately report subcellular biochemical processes through the control of input excitation only (13). Fluorescent nanosensors have been reported for the “A to Z” of analytes, from adenosine triphosphate (ATP) to zinc, and research in this exciting field continues to advance (14–16).
My group’s research focuses on nanosensors relevant to cell and gene therapies. They are for pH (H+) (12) and molecular oxygen (O2) (17) and manufactured from inert polyacrylamide matrices. These nanoparticles (~50 nm in diameter) have been engineered to traverse biological membranes to observe and induce biochemical changes. Our research also has been extended to observe whole-organism bioprocesses.
Subcellular Analytics
Noninvasive targeted subcellular delivery can be considered as the zenith of formulation sciences. That is because cell membranes act as physical barriers, and programmed cellular inertia prevents entry of unknown systems. Research has focused on reducing the effect of membrane barriers (18) using heat shock (19), surfactant treatment (20), electroporation (21), and changes in osmolarity (22). Other methods include accessing known cell-receptor internalization (e.g., clathrin mediated) (23) and using forced entry (e.g., gene-gun delivery) (24). As such, delivery of diagnostics tools such as fluorescent nanosensors has been challenging.
In my group’s strategy, we functionalized fluorescent nanosensors matrices with cationic molecules (Figure 2b–d). Cationic molecules increase the zeta potential of nanoparticle surfaces, which has been hypothesized to facilitate nanoparticle penetration between hydrophobic moieties of lipid bilayers and cause membrane disruption at high concentrations (25). When we control the amount of positive functionalized monomer and nanoparticle concentrations, nanosensors can traverse multiple biological membranes and silently report on key biochemical changes. In separate studies, we have demonstrated how fluorescent nanosensors can be used to characterize subcellular environments for Saccharomyces cerevisiae (26) and human mesenchymal stem cells (hMSCs) (27, 28).
S. cerevisiae has been implemented as an animal-free alternative to using adenoassociated viruses (AAVs) as vectors for gene therapies (29). S. cerevisiae comprises both a cell wall and membrane. The cell wall acts as an additional physical barrier to nanoparticle delivery. Our research has shown that by engineering polyacrylamide-based nanoparticles with the cationic molecule acrylamidopropyl trimethyl ammonium hydrochloride (ACTA), we demonstrated significant improvements in subcellular delivery without obvious changes to S. cerevisiae viability when compared with unfunctionalized particles (26).
We also have shown that fluorescent nanosensors can be used to study the influence of adding carbon sources in the form of sugars such as glucose on intracellular pH. Addition of glucose to S. cerevisiae results in an initial decrease in subcellular pH (after 10 minutes). Subcellular pH recovers to preglucose dose levels after 30 minutes. This research could be translated readily to improve AAV development and manufacturing by optimizing carbon-dose cycles and media replenishment.
hMSCs have been the focus of immense clinical research for their application in cell therapies and regenerative medicine (30). They can differentiate into multiple cell types and replenish their own stem-cell stores (31). Because of their ease of extraction, isolation, and expansion, hMSCs have been used in clinics for treating blood and bone marrow cancers. Through advances in their biomanufacturing, their full potential could be realized (32). Research we have conducted has shown how pH-sensitive nanosensors can be used to monitor the degradation of tools used to facilitate targeted delivery of hMSCs (27) and how light-induced irradiation of internalized nanoparticles could be used to induce biochemical changes and consequential cell differentiation (28).
Silica magnetic-iron nanoparticles (SiMAGs) have been studied for their potential in direct hMSC therapy by using magnetism to facilitate targeted therapy (33). However, the challenges with this strategy are understanding how SiMAGs are processed subcellularly and determining whether they permit long-term magnetic manipulation. Our research investigated whether pH-sensitive fluorescent nanosensors could address those challenges when combined with state-of-the-art technologies such as super-resolution fluorescence microscopy and multicolor flow-cytometric analysis (Figure 2c) (27). Super-resolution fluorescence microscopy confirmed that pH-sensitive nanosensors are colocalized with SiMAGs in lysosomal–endosomal spaces and permit long-term measurement. Flow cytometry was implemented to determine qualitatively and quantitatively the influence of SiMAGs on intracellular pH for seven days. Our analyses suggest that SiMAGs reduce intracellular pH over four days (from pH 5.43 ± 0.06 to 4.81 ± 0.14), which corresponds to SiMAG subcellular processing. The pH recovers to pre-SiMAG dosage levels on day 6 (pH 5.33 ± 0.17). Our studies suggest that SiMAG lysosomal–endosomal pathway exposure degrades both fluorescent silica coatings and iron cores, reducing cell loading by no more than 50% over seven days. That reduction in subcellular SiMAG concentration still would allow long-term magnetic manipulation of hMSCs.
Controlled changes in biochemical processes that produce predifferentiation of hMSCs in vitro afford the prospect of improvements in regenerative efficacy in vivo (34). Our research has shown that controlled changes in subcellular hMSC biochemical processes can be achieved through light-induced generation of reactive oxygen species (ROS), using photoactivatable porphyrins attached to polyacrylamide nanoparticles (Figure 2d) (28). Through control of either the concentration of photoactivatable porphyrins or number of light irradiations, the amount of ROS produced can be regulated. We used a newly synthesized fluorophore to identify ROS generation events through increases in emission intensity. Cytokine analyses, with flow cytometric analysis, indicated light-irradiation–induced controlled apoptotic cell death, rather than uncontrolled necrotic cell death. Our experiments contribute to the development of light-induced differentiation of hMSCs for clinical translation in vitro and for whole-organism analyses.
Whole-Organism Analyses
The potential of fluorescent nanosensors can be demonstrated when they are used to determine real-time in situ analytics for complex bioprocesses in living organisms such as Caenorhabditis elegans (Figure 2e). C. elegans is a free-living soil nematode and the most completely understood animal on the planet in terms of genetics, neurology, and cell survival. Its application as a model in the study of complex biochemical processes has gathered significant momentum. That is because C. elegans is easy to culture (feeds on bacterial lawns on agar plates), has a short life cycle (egg to adult in three days), is optically transparent (permits optical visualization of anatomical events), and has freely available mutants that could function as experimental controls. In addition, because C. elegans nematodes are invertebrates, they do not require ethical approval to conduct research. Therefore, they are an extremely useful model organism in research that combines complex biology such as cell and gene therapy and bioanalytical tools such as fluorescent nanosensors (35).
Our research has shown that pH-sensitive nanosensors can be delivered to C. elegans pharyngeal and intestinal lumen to make measurements (36). C. elegans can be fed nanosensors continually at relatively high concentrations for extended periods in the absence of significant toxicity. Furthermore, nanosensors remain in the C. elegans pharyngeal and intestinal lumen in excess of 24 hours after the challenge has been removed. These findings highlight the inert nature of fluorescent nanosensors and their potential to silently report biochemical changes for extended periods. Measurements of pharyngeal and intestinal pH show that C. elegans acidifies ingested matter such that there is pH gradient from the anterior of the pharynx (5.96 ± 0.31) to the posterior of the intestine (3.59 ± 0.09). Using high-speed fluorescence microscopy and temperature control to reduce the feeding rate, studies have shown that dynamic acidification of intestinal contents can be mapped in real time. These findings suggest that, under optimized conditions, fluorescent nanosensors could be used to monitor the efficacy of cell and gene therapies in situ.
Future Perspective
Fluorescent nanosensors are an extremely powerful tool and have been used to perform inert, long-term high spatial and high temporal measurements in a number of model organisms (8). However, it is important to note that the applications of fluorescent nanosensors to cell and gene therapies are in the early stages, and the true potential is yet to be determined. The tangible benefits of this technology to cell and gene therapies will be realized when measurements of key subcellular molecules and ions demonstrate advantages over extracellular measurement technologies that are essential to biomanufacturing (e.g., time lags and analysis of biochemical processes that occur only subcellularly).
Nevertheless, fluorescent nanosensors’ enhanced measurement capabilities indicate a strong potential to characterize complex biochemical processes upstream, downstream, and in situ for cell and gene therapy manufacture. Furthermore, through the introduction of a new wave of analytical biosensors (37) — which have biodegradable polymeric matrices (38) — advanced production methods (39), and coupled innovative analytical instruments, fluorescent nanosensors are well positioned to enrich biomanufacturing for cell and gene therapies.
Acknowledgment
The author’s work was funded by a Nottingham Research Fellowship from the University of Nottingham, UK.
References
1 Elverum K, Whitman M. Delivering Cellular and Gene Therapies to Patients: Solutions for Realizing the Potential of the Next Generation of Medicine. Gene
Ther. 25 April 2019; https://doi.org/10.1038/s41434-019-0074-7.
2 Approved Cellular and Gene Therapy Products. US Food and Drug Administration: Rockville, MD, 2020; https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products.
3 Chauhan VM. Augmenting Automated Analytics Using Fluorescent Nanosensors. Cell Gene Ther. Insights 4(9) 2018: 837–850; https://insights.bio/cell-and-gene-therapy-insights/journal/articles/augmenting-automated-analytics-using-fluorescent-nanosensors.
4 Luxturna. Spark Therapeutics: Philadelphia, PA, 2020; https://luxturna.com.
5 Kymriah. Novartis: Basel, Switzerland, 2020; https://www.hcp.novartis.com/products/kymriah/acute-lymphoblastic-leukemia-children.
6 Yescarta. Kite Pharma: Los Angeles, CA, 2020; https://www.yescarta.com.
7 Ahmed S, et al. New Generation of Bioreactors That Advance Extracellular Matrix Modeling and Tissue Engineering. Biotechnol. Lett. 41(1) 2019: 1–25; https://doi.org/doi:10.1007/s10529-018-2611-7.
8 Harrison RP, Chauhan VM: Enhancing Cell and Gene Therapy Manufacture Through the Application of Advanced Fluorescent Optical Sensors. Biointerphases 13(1) 2018: 8; https://doi.org/10.1116/1.5013335.
9 Elsutohy MM, et al. Enhanced Distance-Dependent Fluorescence Quenching Using Size-Tuneable Core Shell Silica Nanoparticles. RSC. Adv. 8(62), 2018: 3540–3548; https://doi.org/10.1039/c8ra05929b.
10 Desai AS, et al. Fluorescent Nanosensors for Intracellular Measurements: Synthesis, Characterization, Calibration, and Measurement. Front. Physiol. 4, 2014; https://doi.org/10.3389/fphys.2013.00401.
11 Chauhan VM, Giuntini F, Aylott, JW. Quadruple Labeled Dual Oxygen and pH-Sensitive Ratiometric Nanosensors. Sens. Bio-sens. Res. 8, 2016: 36–42; https://doi.org/10.1016/j.sbsr.2016.03.007.
12 Chauhan VM, Burnett GR, Aylott JW. Dual-Fluorophore Ratiometric pH Nanosensor with Tuneable pK(a) and Extended Dynamic Range. Analyst 136(9) 2011: 1799–1801; https://doi.org/10.1039/c1an15042a.
13 Chauhan VM. Development of Fluorescent Nanosensors for the Measurement of pH: Molecular Oxygen and Temperature in Biological Systems. University of Nottingham: Nottingham, United Kingdom, 2014.
14 Ozalp VC, Nielsen LJ, Olsen LF. An Aptamer-Based Nanobiosensor for Real-Time Measurements of ATP Dynamics. Chembiochem. 11(18), 2010: 2538–2541; https:// doi.org/10.1002/cbic.201000500.
15 Sumner JP, et al. A Fluorescent PEBBLE Nanosensor for Intracellular Free Zinc. Analyst 127(1) 2002: 11–16; https://doi.org/10.1039/B108568A.
16 Rong GX, et al. Recent Developments in Nanosensors for Imaging Applications in Biological Systems. Annual Review of Analytical Chemistry, Volume 12. Bohn PW, Pemberton JE, Eds. Annual Reviews: Palo Alto, CA, 2019; https://doi.org/10.1146/annurev-anchem-061417-125747.
17 Giuntini F, et al. Conjugatable Water-Soluble Pt(II) and Pd(II) Porphyrin Complexes: Novel Nano- and Molecular Probes for Optical Oxygen Tension Measurement in Tissue Engineering. Photochem. Photobiol. Sci. 13(7) 2014: 1039–1051; https://doi.org/10.1039/c4pp00026a.
18 Vaara M. Agents that Increase the Permeability of the Outer Membrane. Microbiol. Rev. 56(3), 1992: 395–411; https://doi.org/10.1128/mmbr.56.3.395-411.1992.
19 Piper PW, et al. Hsp30, the Integral Plasma Membrane Heat Shock Protein of Saccharomyces cerevisiae, Is a Stress-Inducible Regulator of Plasma Membrane H+–ATPase. Cell Stress Chaperones 2(1) 1997: 12–24.
20 Valli M, et al. Intracellular pH Distribution in Saccharomyces cerevisiae Cell Populations, Analyzed By Flow Cytometry. Applied Environ. Microbiol. 71(3) 2005: 1515–1521; https://doi.org/10.1128/aem.71.3.1515-1521.2005.
21 Poulsen AK, et al. Probing Glycolytic and Membrane Potential Oscillations in Saccharomyces cerevisiae. Biochem. 47(28) 2008: 7477–7484; https://doi.org/10.1021/bi800396e.
22 Miyazaki J, et al. Adhesion and Internalization of Functionalized Polystyrene Latex Nanoparticles Toward the Yeast Saccharomyces cerevisiae. Advanced Powder Technol. 25(4) 2014: 1394–1397; https://doi.org/10.1016/j.apt.2014.06.014.
23 Xu S, et al. Targeting Receptor-Mediated Endocytotic Pathways with Nanoparticles: Rationale and Advances. Advan. Drug Deliv. Rev. 65(1) 2013: 121–138; https://doi.org/10.1016/j.addr.2012.09.041.
24 Ahmed S, et al. Freezing-Assisted Gene Delivery Combined with Polyampholyte Nanocarriers. ACS Biomat. Sci. Engin. 3(8) 2017: 1677–1689; https://doi.org/10.1021/acsbiomaterials.7b00176.
25 Tatur S, et al. Effect of Functionalized Gold Nanoparticles on Floating Lipid Bilayers. Langmuir 29(22) 2013: 6606–6614; https://doi.org/10.1021/la401074y.
26 Elsutohy MM, et al. Real-Time Measurement of the Intracellular pH of Yeast Cells During Glucose Metabolism Using Ratiometric Fluorescent Nanosensors. Nanoscale 9(18) 2017: 5904–5911; https://doi.org/10.1039/c7nr00906b.
27 Harrison RP, et al. Intracellular Processing of Silica-Coated Superparamagnetic Iron Nanoparticles in Human Mesenchymal Stem Cells. RSC Advan. 9(6) 2019: 3176–3184; https://doi.org/10.1039/c8ra09089k.
28 Lavado AS, et al. Controlled Intracellular Generation of Reactive Oxygen Species in Human Mesenchymal Stem Cells Using Porphyrin Conjugated Nanoparticles. Nanoscale 7(34), 2015; https://doi.org/10.1039/C5NR00795J.
29 Barajas D, et al. Generation of Infectious Recombinant Adenoassociated Virus in Saccharomyces cerevisiae. Plos One 12(3) 2017: e0173010; https://doi.org/10.1371/journal.pone.0173010.
30 Meirelles LD, et al. Mechanisms Involved in the Therapeutic Properties of Mesenchymal Stem Cells. Cytokine Growth Factor Rev. 20(5–6), 2009: 419–427; https://doi.org/10.1016/j.cytogfr.2009.10.002.
31 Nombela-Arrieta C, Ritz J, Silberstein LE. The Elusive Nature and Function of Mesenchymal Stem Cells. Nature Rev. Mol. Cell Biol. 12(2) 2011: 126–131; https://doi:10.1038/nrm3049.
32 Olsen TR, et al. Peak MSC — Are We There Yet? Front. Med. 5, 2018: 178; https://doi.org/10.3389/fmed.2018.00178.
33 Landazuri N, et al. Magnetic Targeting of Human Mesenchymal Stem Cells with Internalized Superparamagnetic Iron Oxide Nanoparticles. Small 9(23) 2013: 4017–4026; https://doi.org/10.1002/smll.201300570.
34 Baksh D, Yao R., Tuan RS. Comparison of Proliferative and Multilineage Differentiation Potential of Human Mesenchymal Stem Cells Derived from Umbilical Cord and Bone Marrow. Stem Cells 25(6) 2017: 1384–1392; https://doi.org/10.1634/stemcells.2006-0709.
35 Lightfoot JW, et al. Comparative Transcriptomics of the Nematode Gut Identifies Global Shifts in Feeding Mode and Pathogen Susceptibility. BMC Research Notes 9(1) 2016: 142.
36 Chauhan VM, et al. Mapping the Pharyngeal and Intestinal pH of Caenorhabditis Elegans and Real-Time Luminal pH Oscillations Using Extended Dynamic Range pH-Sensitive Nanosensors. ACS Nano 7(6) 2013: 5577–5587; https://doi.org/10.1021/nn401856u.
37 Chauhan VM, et al. Gold-Aptamer-Nanoconstructs Engineered to Detect Conserved Enteroviral Nucleic Acid Sequences. ChemRxiv 23 June 2019; https://doi.org/10.26434/chemrxiv.8312324.v1.
38 Al-Natour MA, et al. Facile Dye-Initiated Polymerization of Lactide–Glycolide Generates Highly Fluorescent Poly(lactic-co-glycolic Acid) for Enhanced Characterization of Cellular Delivery. ACS Macro Lett. 9(3) 2020: 431–437; https://doi.org/10.1021/acsmacrolett.9b01014.
39 Martins C, et al. Modeling Protein Therapeutic Coformulation and Codelivery with PLGA Nanoparticles Continuously Manufactured by Microfluidics. React. Chem. Eng. 5, 2020: 308–315; https://doi.org/10.1039/C9RE00395A.
Veeren M. Chauhan, PhD, is a Nottingham Research Fellow in the Bioinspired Therapeutics Advanced Materials and Healthcare Technologies group at the University of Nottingham, School of Pharmacy, Boots Science Building, Science Road, Nottingham, NG7 2RD United Kingdom; [email protected]; www.veerenchauhan.com. The author declares no conflict of interest.
You May Also Like





