- Sponsored Content
Single-Use Systems: Balancing the Need Between Customization and Standardization
August 10, 2016
Sponsored by Parker Domnick Hunter
 In June 2016, Parker domnick hunter began manufacturing in its new single-use facility in Birtley, Co Durham, UK. Here I discuss the approach taken in the design, testing, and manufacturing of single-use assemblies and some of the advantages they can have in terms of lead times, inventory control, and supply chain robustness.
In June 2016, Parker domnick hunter began manufacturing in its new single-use facility in Birtley, Co Durham, UK. Here I discuss the approach taken in the design, testing, and manufacturing of single-use assemblies and some of the advantages they can have in terms of lead times, inventory control, and supply chain robustness.
Challenges of Customization
For some time, the message relayed to biopharmaceutical manufacturers was to design exactly what you want and need for each process. That meant a customized solution for every operation, for every scale, and for every product. There is no doubt that single-use technology has dramatically increased the flexibility of biopharmaceutical manufacturing facilities, but this has led to some challenges for the implementation of single-use systems.
High levels of customization can mean that organizations have to stock and control a large number of low-volume items, including managing and (where necessary) writing off expired stock. In addition, the requirement for lots of small-volume production runs can increase lead times for products.
Ultimately, the time required for designing, testing, and managing single-use systems will potentially result in longer implementation times and increased cost of goods.
Customization or Configuration
Parker domnick hunter believes that all such challenges can be addressed and managed in such a way that enables single-use assemblies to be manufactured in a robust and repeatable process in the quantities needed. The solution is standardization.
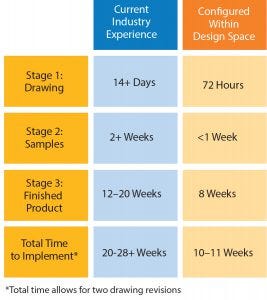
Figure 1: Comparison of lead times
There will always be a need for flexibility in single-use design. In fact, the days of processing being made to fit into a plant should be long gone. However, as an industry, we should move away from custom solutions to configured solutions based within a validated design space. If we are willing to make that compromise, we can gain three things: time, performance, and quality.
With the development of a dynamic but controlled design space and manufacturing capacity in an ISO Class 7 cleanroom at our UK facility, configured solutions can be rapidly designed, tested, and manufactured to a consistently high standard. Figure 1 shows the effect this can have on assembly lead times compared with the industry standard.
Conclusion
Single-use technology is enabling biopharmaceutical products to be brought to market faster and more safely than ever before. As the industry matures, the need for standardization of systems, components, and designs along with manufacturing and testing methods will deepen.
An end user will always own its process, but a single-use supplier must be responsible for ensuring that a product is fit for purpose. This applies both from a performance and regulatory point of view, and to ensure that a product can be manufactured to the same quality and time line today, tomorrow, and for years to come.
Standardization offers significant time saving and keeps an end user’s focus where it should be: process development and the production of life-saving biopharmaceuticals.
Guy Matthews is global market development manager at Parker domnick hunter Process Filtration, Durham Road, Birtley, County Durham, DH3 2SF, UK; 44-191-410 5121; [email protected]; www.parker.com/dh-bioprocessing.
You May Also Like





