Since January 2016 (with a brief interlude as described below), the Patent Trial and Appeal Board has been attempting to adjudicate the proper inventorship of CRISPR technology in (to date) six separate patent-interference proceedings. (Scientific “priority has been decided, for now, by the awarding of the Nobel Prize in Chemistry to Jennifer Doudna and Emmanuelle Charpentier in 2020; see the “Priority Claims” box). CRISPR-based gene editing was hailed as the “Breakthrough of the Year” in 2015 (1), and the scientific community has issued equally high praise for the technology’s power and potential implications. These factors have made the outcomes of the interference cases fraught with economic benefits for the eventual victors — for both the scientists and the assignees of CRISPR-associated patents and applications. (Note: Just before this article went to press, the Patent Trial and Appeal Board [PTAB] ruled that between inventors from the Broad Institute and the University of California/Berkeley [and each party’s colleagues], the Broad inventors deserved priority of invention (as discussed herein.)]
CRISPR Technology
Clustered regularly interspaced short palindromic repeats (CRISPR) technology is used as a system for altering chromosomal sequences in situ in cells. CRISPR occurs naturally in bacteria as an “immune system” for preventing infection with bacterial phage (viruses) (2). It combines an endonuclease (CRISPR-associated protein 9, Cas9) with two short RNA molecules, one of which is complementary to a sequence that forms a virus target genome.
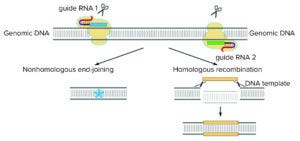
Figure 1: CRISPR provides a mechanism for inserting or deleting specific DNA sequences using CRISPR-associated targeting RNAs and the Cas9 RNA-guided DNA endonuclease enzyme.
As first developed by Doudna and Charpentier, this natural system can be adapted to cleave any DNA sequence. Just as the discovery of bacterial restriction enzymes by Herbert Cohen and Stanley Boyer in 1972 provided the ability to dissect DNA at specific sites in its sequence (3), CRISPR provides a mechanism for inserting or deleting specific DNA sequences using CRISPR-associated targeting RNAs and the Cas9 RNA-guided DNA endonuclease enzyme. The technology has provided for the first time the type of specificity to alter DNA that the polymerase chain reaction (PCR) provided a generation ago for amplifying specific DNA, as illustrated in Figure 1.
Patent Interferences
Until the enactment of the Leahy–Smith America Invents Act in 2012, the US patent system granted patents to the first person to have conceived an invention. Under the old law, when more than one inventor or group of inventors applied for a patent, the US Patent and Trademark system instituted a proceeding called an interference to determine which inventor or group of inventors was the first to invent. The interference declaration names one party as a senior party and the other as a junior party based on which has the earliest patent filing date. These designations are important: Without evidence to the contrary, the board considers the senior party to be the presumptive inventor; thus, the junior party bears the burden to establish prior right based on an earlier invention. The patents and patent applications involved in the several interferences involving CRISPR technology were filed under this regime (with certain disputes as discussed below).
An interference proceeds in two stages. In the first stage, the parties present motions that can modify the count (an artificial claim that determines the metes of a disputed invention), declare that certain claims are outside the scope of the count (or vice versa), and ask for a finding that the claims are invalid under any provisions of the patent statute. If such motions are not decided in a way that would disqualify one or both parties, then the interference moves on to a second stage, in which a junior party (having the later filing date) will present its proofs of conception and reduction to practice, and the senior party will be permitted to oppose.
Reduction to practice entails the actual construction of an item or performance of process steps for a patent (or in the alternative, filing a patent application that sufficiently describes the invention, termed a constructive reduction to practice). The senior party is under no obligation to present proofs earlier than its earliest filing date unless the junior party provides evidence of (at least) having earlier conceived of the invention (even without reducing it to practice). Usually both parties submit evidence of their earliest conception and reduction to practice by filing a patent application.
Office rules mandate the times for these two stages. An interference usually concludes in a decision within 36 months of the declaration. Alternatively, The parties can settle an inference privately. In such cases, a losing party files a “Concession of Priority,” and the prevailing party (usually) grants a license to the loser; such settlement agreements are kept confidential, but they must be filed with the USPTO. Otherwise, everything else in an interference is public information that can be found under the PTAB on the US Patent and Trademark Office’s (USPTO’s) Patent Application Information Retrieval (PAIR) website. Office rules mandate the times for these two stages. An interference usually concludes in a decision (if not settled earlier) within 36 months of the declaration.
Parties to the CRISPR Interferences: Four inventor groups are involved in the CRISPR interferences.
CVC: Jennifer Doudna and Martin Jinek (University of California, Berkeley); and Emmanuelle Charpentier and Krzysztof Chylinski (University of Vienna).
Broad: Feng Zhang (the Broad Institute, Massachusetts Institute of Technology); and Randall J. Platt, Neville E. Sanjana, Patrick Hsu, Le Cong, and Fei Ran (Harvard University)
ToolGen, Inc: Jin-Soo Kim, Seung Woo Cho, and Sojung Kim
Sigma-Aldrich Co. LLC: Fuqiang Chen and Gregory D. Davis.
The four key interferences are summarized below. All are between the above parties in different combinations, and all are pending except for the first one, Interference No. 106,048, which the PTAB resolved on the grounds that there was no interference-in-fact, for the reasons set forth below.
Resolving Interference-in-Fact (No. 106,048, 11 January 2016): The USPTO named Feng Zhang and his colleagues (the named inventors from the Broad Institute/MIT/Harvard patents) as the junior party and Jennifer Doudna and her colleagues at UC–Berkeley as senior party (4).
The interference declaration included all claims of all CRISPR patents owned by the Broad group (Broad, Harvard, and MIT), whereas the CVC (Berkeley) group involved only one pending application. The declaration set forth the following count to define the interfering subject matter. (A count is a description of the interfering subject matter that sets the scope of admissible proofs on priority. When there is more than one count, each count must describe a patentably distinct invention.) Of importance for issues that arose in later interferences, this count encompasses dual-molecule RNA species — wherein the guide RNA (gRNA) and transactivating CRISPR RNA (tracrRNA) are distinct RNA molecules — and single-molecule RNA species, wherein the tracrRNA and gRNA are linked, typically with an oligonucleotide spacer called a single-guide (sg) RNA. Italics indicate those relevant issues and limitations:
Despite several preliminary motions, the PTAB granted Broad’s motion that there was no interference-in-fact between several patents and patent applications owned by the Broad Institute and those owned by the Regents of the University of California, Berkeley. That decision ended the interference without prejudice to any of the claims corresponding to the interference count. Accordingly, both parties could license (and assert) their patents against any third party.
The grounds for the board’s decision (affirmed by a Federal Circuit on appeal) were that the interference failed the test under the patent rules that one party’s claims, if considered to be prior art, would have “anticipated or rendered obvious” the subject matter of the other party. If “prior art” is established in a patent claim, it would mean that a second party would derive from that an “expectation of success” when duplicating the work — making the possibility of success “obvious.”
The question before the board was whether the evidence presented by the parties established that the UC claims, if prior art, would have rendered Broad’s claims obvious. The board concluded that they would not have and hence that there was no interference-in-fact between the parties’ claims.
Assessing Prior Art — Establishing Reasonable Expectation of Success: In considering the evidence before it, the PTAB gave great weight to cautious, contemporaneous statements related to Doudna’s disclosure of in vitro CRISPR activity regarding whether the system would work in eukaryotic cells. Specifically, Doudna’s statements convinced the board that although the results “suggested the ‘exciting possibility’” that CRISPR-Cas9 could be operative in eukaryotic cells, “it was not known whether such a bacterial system would function in eukaryotic cells.” And “[i]n another report, Doudna was quoted as stating that she had experienced ‘many frustrations’ getting CRISPR to work in human cells and that she knew that if she succeeded, CRISPR would be ‘a profound discovery.’”
UC’s assertion of other statements by their inventors that could be interpreted more positively did not convince the board that there was a reasonable expectation of success in the art for getting the CRISPR-Cas9 system to work in eukaryotic cells. The board stated that “even UC’s own expert in the interference voiced (contemporaneous) skepticism regarding whether a skilled worker would have had an expectation of success that the invention would be operative in eukaryotic cells in view of inventor Doudna’s success in vitro.” As it turned out, several groups (including those involved in other, later-instituted interferences) were successful in practicing CRISPR in eukaryotic cells, but the board held the following:
Focusing on Success with Eukaryotic Cells (No. 106,115, 24 June 2019): After failing to prevail in the above (048) interference, the California group returned to the patent office. It introduced claims in pending applications directed specifically to eukaryotic cell embodiments of CRISPR. The PTAB issued a declaration of interference between UC Berkeley, the University of Vienna, and Emmanuelle Charpentier (abbreviated to “CVC” hereafter) as junior party and the Broad Institute, Massachusetts Institute of Technology, and Harvard University (“Broad”) as senior party (5). The count in this interference was a hybrid one that included the relevant limitations contained in the previous applications, the only difference being that the portion corresponding to CVC’s claim affirmatively recites the sgRNA species:
The parties proposed preliminary motions, some of which the board granted leave to be filed, and in due course the board ruled on those motions (none of which substantially changed the posture of the interference). The consequences of those decisions were that the interference proceeded to the priority phase, and Broad’s status as senior party was unchanged; accordingly, CVC had the burden of showing earlier conception of the subject matter defined in the count as declared.
In the priority phase, junior party CVC provided its evidence of conception and reduction to practice. Their proofs were based on the CVC inventors’ conceiving of CRISPR embodiments for which the sequence-specific crRNA (which recognizes the site for DNA cleavage) was linked to the structural tracrRNA by a short oligonucleotide sequence as set forth in a laboratory notebook on 1 March 2012 (Figure 2).
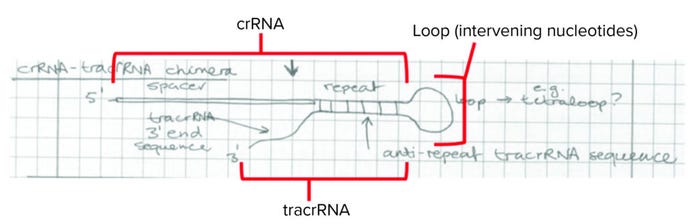
Figure 2: A laboratory notebook entry presented as evidence of conception and reduction to practice by the University of California, Berkeley; the University of Vienna; and Emmanuelle Charpentier (crRNA = CRISPR RNA, tracrRNA = transactivating CRISPR RNA).
The priority statement also set forth evidence of further “serial events of additional conception” occurring in August, October, and November 2012 and its assertions that it pursued reduction to practice with the statutorily required diligence and that it never abandoned, suppressed, or concealed its invention.
Broad also filed a priority statement (which, as senior party, it was not obliged to do). The statement compared the timelines for Broad’s own conception and reduction to practice with CVC’s (Figure 3). In addition, Broad argued that the board also could consider evidence of Zhang’s earlier “successful eukaryotic experiments with his dual-molecule RNA CRISPR-Cas9 systems.” Those experiments did not directly link crRNA and tracrRNA. The motion concludes with assertions of diligence and that Zhang did not abandon, suppress, or conceal Broad’s invention, based among other things on a 5 October 2012 publication in Science (6) and Broad’s provisional application filing on 12 December 2012 (that antedated CVC’s earliest filing date). In Broad’s telling of the tale, CVC’s asserted dates of reduction to practice were reinterpreted to show failure to achieve CRISPR in eukaryotic cells.
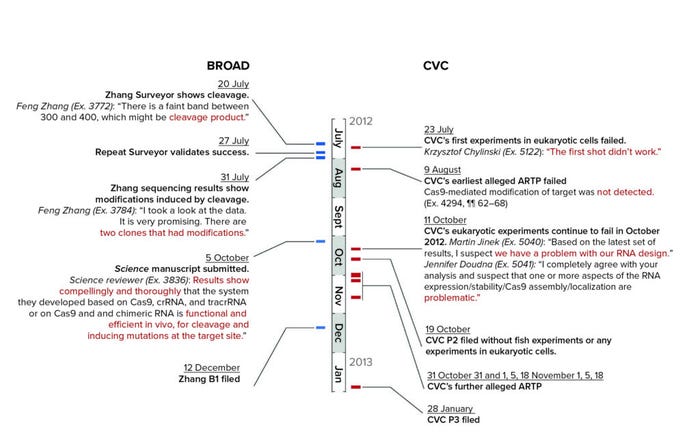
Figure 3: Key dates of CRISPR interferences; “B” codes refer to utility patent grants (B1 = no pre-grant publication); “P” codes refer to stages of plant patent grants (see also https://www.finnegan.com/en/insights/blogs/prosecution-first/the-abcs-of-patent-kind-codes.html). (ARTP = prior art; Surveyor = Broad’s system for detecting CRISPR-mediated cleavage).
In its priority statement, CVC included two statements from Broad scientists, arguing in a separate motion that the Broad inventors had derived their eukaryotic CRISPR invention from In its priority statement, CVC included two statements from Broad scientists, arguing in a separate motion that the Broad inventors had derived their eukaryotic CRISPR invention from CVC. The first statement was by Luciano Marraffini, who collaborated with Broad’s Feng Zhang and cofiled Broad’s first provisional application, saying that the idea for sgRNA originated from the Jinek 2012 paper:
The second statement highlighted a 2015 email to Doudna from Shuailiang Lin, another collaborator of Zhang’s and coinventor on Broad’s first provisional application:
Based on this evidence, CVC requested and the board granted leave for them to depose Dr. Marraffini. CVC argued that Broad inventor Zhang derived the claimed invention from disclosure of CVC’s conception from Dr. Marraffini. He knew about CVC’s invention because he had been a confidential reviewer of the manuscript later published in Science by Jinek et al (6). Dr. Marraffini also attended a CRISPR conference at Berkeley on 26 June 2012 at which the Doudna laboratory disclosed its CRISPR findings. The brief compares what was disclosed at the meeting, what Dr. Marraffini disclosed to Zhang, and what Zhang communicated to his colleague Cong, first author on the paper published in the January 2013 issue of Science and containing Broad’s disclosure of CRISPR practiced in eukaryotic cells (7) (Figure 4).
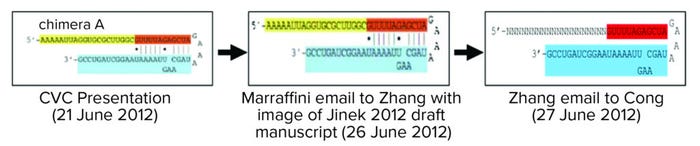
Figure 4: Timeline of disclosures related to Science publications.
According to CVC, Dr. Marraffini had immediately communicated the key finding disclosed at that meeting by the Doudna and Charpentier group: that CRISPR could be performed by single-guide RNA (sgRNA) comprising tracrRNA sequence-specific mature crRNA fragments covalently linked in association with the Cas9 protein. CVC also relied on Dr. Marraffini’s testimony as evidence that, before this disclosure, Zhang understood that formation of functional CRISPR complexes required RNase III cleavage of unprocessed tracrRNA and crRNA separately.
CVC’s derivation argument rests crucially on Dr. Marraffini’s testimony. Before he attended the 2012 CRISPR Conference, he already had reviewed CVC’s draft manuscript as one of the peer-review referees for Science. CVC submitted the draft to that publication by 8 June 2012, just before the conference. The manuscript revealed to Dr. Marraffini that processed tracrRNA, mature crRNA, and Cas9 are the necessary and sufficient components of the catalytic CRISPR-Cas9 DNA-cleavage complex, elements that clarified/enabled the design and use of a functional sgRNA CRISPR-Cas9 system. The paper also expressly stated that the sgRNA CRISPR-Cas9 system could be used for “genome editing in cells of the three kingdoms of life for biotechnological, biomedical, and gene therapeutic purposes” (6).
Based on Dr. Marraffini’s testimony, CVC set out its own summary timeline of events by the parties, modifying the timeline presented in Broad’s priority motion according to CVC’s version of events as supported by the evidence set forth in this brief (Figure 5). Finally, CVC asked the board to address accusations of inequitable conduct against Broad. CVC alleges that “Broad made at least one affirmative material misstatement during prosecution of each of Broad’s involved patents, applications, or parent applications to which they claim priority” — specifically, in a declaration by named inventor Zhang regarding actual reduction to practice of CRISPR-Cas9 in eukaryotic cells before May 2012. CVC asserted that Broad’s statements were untruthful because the CRISPR system did not include tracrRNA, which is necessary for CRISPR to be functional. CVC also asserted that Zhang’s alleged “conception” arose only after reading a Berkeley prior-art disclosure. If the board finds that inequitable conduct arose in prosecution of the Broad patents, some or all of them could be held to be unenforceable.
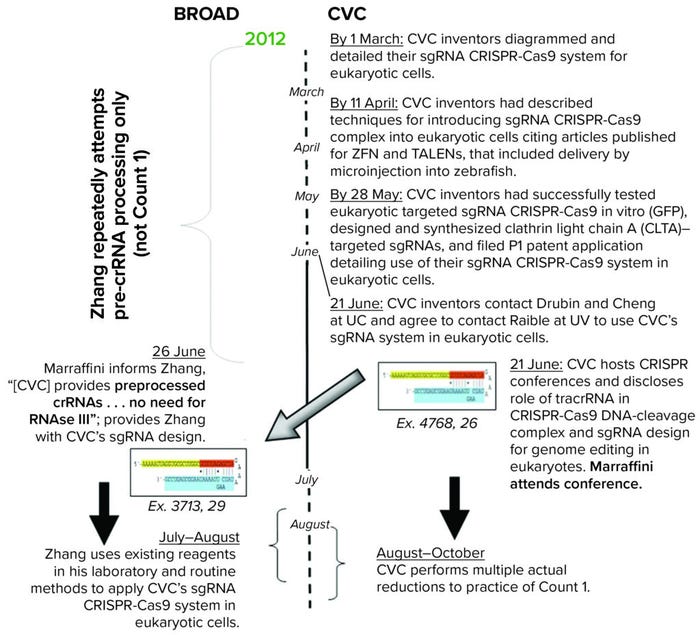
Figure 5: CVC’s modified timeline based on Marraffini’s testimony (ZFNs = zinc finger nucleases; TALENs = transcription activator-like effector nucleases).
On 28 February 2022, the PTAB granted priority for eukaryotic CRISPR to the Broad Institute against CVC. The board was convinced by Broad’s arguments that CVC’s attempts to reduce eukaryotic CRISPR to practice were ineffective until after Broad’s reduction to practice, as evidenced by a manuscript submitted on 5 October 2012. The board operated on the legal principle that “priority of invention goes to the first party to reduce an invention to practice unless the other party can show that it was the first to conceive of the invention and that it exercised reasonable diligence in later reducing that invention to practice” (8). The board was unconvinced that CVC’s 1 March 2012 conception satisfied the requirements of “complete” conception. Broad persuasively argued that the evidence of CVC’s attempts to reduce eukaryotic CRISPR to practice showed sufficient uncertainty and failures to render incomplete CVC’s conception.
ToolGen’s Request for Priority Benefit (Nos. 106,126 and 106,127, 14 December 2020: Separate interferences (9, 10) were declared between ToolGen Inc. as senior party and Broad (Interference No. 106,126) and CVC (Interference No. 106,127) as junior parties. ToolGen is a Korean biotechnology company focused on translating CRISPR-Cas9 technology for therapeutics and agricultural technology. ToolGen is seeking priority benefit based on its US Patent Application Publication No. US20150344912 (see the “Priority Benefits” box). Decisions are unlikely to be handed down before summer of 2022.
Sigma-Aldrich Narrows the Scope (Nos. 106,132 and 106,133, 21 June 2021): The board declared separate interferences (11, 12) between Sigma-Aldrich Co. LLC as senior party and CVC (Interference No. 106,132) and Broad (Interference No. 106,133) as junior parties. The Sigma-Aldrich portion of the count differs from all other counts by reciting as an affirmative limitation that the Cas9 protein is linked to only one nuclear localization signal (NLS) and that the sequence encoding it is codon-optimized; the “protospacer” between the gRNA and the tracrRNA comprises a “protospacer adjacent motif (PAM)”; and “the CRISPR-Cas type II protein introduces a double-stranded break at the target site, and repair of the double-stranded break by a DNA homology-directed repair (HDR) process leads to integration or exchange of the donor sequence into the chromosomal sequence.” That portion of the count is limited to insertion of a heterologous DNA sequence at the CRISPR cleavage site. Sigma-Aldrich’s claims are directed to CRISPR embodiments that insert heterologous DNA into specific genomic DNA sites in eukaryotic cells. Although the same embodiments also are encompassed in CVC’s, Broad’s, and ToolGen’s claims, the counts in those interferences do not recite embodiments so limited.
Preliminary motions have been authorized, and briefing by the parties is ongoing, with decisions unlikely to come before fall of 2022.
Future Prospects
In view of the board’s 28 February 2022 decision, the status of eukaryotic CRISPR is where the parties left it after the ’048 Interference decision but with Broad in a decidedly better position, having been granted priority on the merits here. All Broad’s patents and applications in interference remain in force and effect, and CVC’s applications containing claims directed to eukaryotic CRISPR are finally rejected for lack of priority. The board was careful to note that CVC retained its patents on CRISPR without any cell-specific limitations, but that is almost certainly ephemeral with regard to eukaryotic CRISPR; CVC should be estopped from asserting those claims against Broad’s licensees (or anyone else) practicing eukaryotic CRISPR. It can be expected that CVC will ask for reconsideration from the board and if, as can be expected, the request is denied, appeal to the Federal Circuit.
All this means is that the provenance of eukaryotic CRISPR has not been settled for good by the board’s decision. But should Broad prevail and the Federal Circuit affirm, it is likely that interferences between CVC and (separately) ToolGen and Sigma-Aldrich will be dissolved.
So far, the interference challenges have not impeded development of CRISPR technology materially, whether used by basic research scientists (predominantly in universities) or private companies. If (or when) the interferences have established which of the parties deserves inventorship of this technology may either provoke aggressive enforcement of the resulting patent rights or (perhaps less likely) increase the impetus for broader licensing policies.
Finally, the possibility cannot be ignored that Broad will prevail on priority in the ‘115 interference but that CVC’s allegations of inequitable conduct will render Broad’s patent estate unenforceable. The consequences of such an outcome are beyond the scope of this paper but tantalizing to contemplate.
References
1 Travis J. Breakthrough of the Year: CRISPR Makes the Cut. Science News 17 December 2015; https://www.science.org/content/article/and-science-s-2015-breakthrough-year.
2 Horvath P, Barrangou R. CRISPR/Cas, the Immune System of Bacteria and Archaea. Science 327(5962) 2010: 167–170; https://doi.org/10.1126%2FScience.1179555.
3 Cohen SN, et al. Construction of Biologically Functional Bacterial Plasmids In Vitro. Proc. Nat. Acad. Sci. (USA) 70(11) 1973: 3240–3244; https://doi.org/10.1073%2Fpnas.70.11.3240.
4 Patent Interference No. 106,048: The Broad Institute, Inc., et al. v. Regents of the University of California et al. US Patent and Trademark Office Patent Trial and Appeal Board: Alexandria, VA, 11 January 2016; https://patentdocs.typepad.com/files/declaration.pdf.
5 Patent Interference No. 106,115: Regents of the University of California et al. v. the Broad Institute, Inc., et al. US Patent and Trademark Office Patent Trial and Appeal Board: Alexandria, VA, 24 June 2019; https://www.broadinstitute.org/files/news/pdfs/106115-NoticeDeclaringInterference.pdf.
6 Jinek M, et al. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 337(6096) 2012: 816–821; https://doi.org/10.1126/science.1225829.
7 Cong L, et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 339(6121) 2013: 819–823; https://doi.org/10.1126/science.1231143.
8 Patent Interference No. 101,100: Cooper v. Goldfarb. US Court of Appeals, Federal Circuit: Washington, DC, 2 March 2001; https://caselaw.findlaw.com/us-federal-circuit/1421492.html.
9 Patent Interference No. 106,126: The Broad Institute, Inc., et al. v. ToolGen, Inc. US Patent and Trademark Office Patent Trial and Appeal Board: Alexandria, VA, 14 December 2020; https://patentdocs.typepad.com/files/order-29.pdf.
10 Patent Interference No. 106,127: Regents of the University of California, et al. v. ToolGen, Inc. US Patent and Trademark Office Patent Trial and Appeal Board: Alexandria, VA, 14 December 2020; https://patentdocs.typepad.com/files/motion-to-exclude.pdf.
11 Patent Interference No. 106,132: Regents of the University of California, et al. v. Sigma-Aldrich Co., LLC. US Patent and Trademark Office Patent Trial and Appeal Board: Alexandria, VA, 21 June 2021; https://www.pbwt.com/content/uploads/2019/10/2019.07.19-Sigma-Aldrich-CRISPR-petition.pdf.
12 Patent Interference No. 106,133: The Broad Institute, Inc., et al. v. Sigma-Aldrich Co., LLC. US Patent and Trademark Office Patent Trial and Appeal Board: Alexandria, VA, 21 June 2021; https://patentdocs.typepad.com/files/broad-motion-3-1.pdf.
Kevin E. Noonan, PhD, is a partner with McDonnell Boehnen Hulbert & Berghoff LLP, 300 South Wacker Drive #3100, Chicago, IL 60606; [email protected].












