 The motto of the European Serum Products Association (ESPA) — “Serum Saves Lives” — reaffirms the essential role that animal serum plays in cell-culture–based research and applications to protect the health of both human and animal populations. Animal serum, especially fetal bovine serum (FBS), needs to be available in abundant supply and at affordable prices to medical and veterinary facilities all over the world. With one billion cattle globally, the supply of FBS should be plentiful, but not all countries qualify as producers for the international market.
The motto of the European Serum Products Association (ESPA) — “Serum Saves Lives” — reaffirms the essential role that animal serum plays in cell-culture–based research and applications to protect the health of both human and animal populations. Animal serum, especially fetal bovine serum (FBS), needs to be available in abundant supply and at affordable prices to medical and veterinary facilities all over the world. With one billion cattle globally, the supply of FBS should be plentiful, but not all countries qualify as producers for the international market.
The animal-health status and regulatory infrastructure of countries differ around the world, as does their ability to control and eradicate diseases. Those factors are influenced by the size of the country, its infrastructure of roads and communication, bordering countries, the cattle population and average herd size, and the number of veterinarians employed in the public sector — among other things. To be approved as FBS suppliers, exporting countries must meet stringent requirements relating to their regulatory veterinary infrastructure, animal-health status, disease-control programs, meat-inspection programs, and market access for beef exports. Without the backing of well-developed animal-health programs and related activities, reliable and safe bovine serum could not be made available.
Despite the widely varying characteristics and conditions that exist among countries, the animal-health regulatory infrastructures of FBS-exporting countries are tailor-made to meet the import requirements of their trading partners based on international standards of the World Organization for Animal Health (OIE). We believe that there is no basis for commercially motivated claims that the regulatory infrastructures of some FBS-exporting countries are superior to those of others.
A Double-Standard Market
FBS harvesting began in the United States in the 1960s, and for over a decade that was the only source. In the 1970s and 1980s, FBS from Canada, Mexico, Oceania, and Central America became available to the US market and the rest of the world. European and South American sources for FBS became available to the international market in the 1990s, but the US Department of Agriculture (USDA) decided not to authorize additional sources of FBS. The inability of European and South American serum to access the US market resulted in the formation of two different market-segments:
USDA-approved FBS with limited supply and very high prices
Serum that is not USDA approved, but with abundant supply and affordable prices.
The USDA ban on additional sources restricts access to FBS for researchers and pharmaceutical companies in the United States and creates economic incentives for falsification of serum origin. Subsequent cases of falsified origin have plagued the serum industry for years (1–3).
The USDA has made three attempts to authorize additional sources of FBS: one in 1994 (4), a second in 2006 (5), and a third initiated in 2014. The first two proposals were withdrawn or abandoned because of opposition from the US serum industry; the third (after six years) continues in risk assessment, with the final outcome yet to be determined as of January 2021.
Scientifically incorrect claims continue to be publicized as justification of price differences for serum based on the “animal-health status” and “regulatory infrastructure” of FBS-producing countries (6). A number of recent scientific publications sponsored by ESPA and the International Serum Industry Association (ISIA) have shown that there is no basis for these claims (7, 8).
International Standards for Veterinary Regulatory Services
The OIE, with the help of its 182 member countries, has developed and approved international standards for Animal Health Regulatory Services. These standards are published in two documents for land-dwelling animals (9, 10). Volume one of the Terrestrial Animal Health Code establishes specific standards for the quality of veterinary services, animal disease prevention (surveillance, diagnosis, notification, and control), self-declaration of disease status, OIE official recognition of disease status, import risk analysis, international veterinary certifications, trade measures and procedures, and animal welfare (9). Volume two of the code provides disease-specific standards designed to prevent the introduction of diseases into importing countries. The second document, the OIE manual, contains standards for veterinary diagnostic laboratories along with available tests and vaccine standards specific for 166 diseases that have trade implications for land-dwelling animals (10).
The ability of regional veterinary services in individual countries to implement the OIE standards determines to a great extent whether those countries can meet the rigorous requirements of importers. To help member countries evaluate their own capabilities, the OIE has developed an evaluation tool based on international standards for measuring the performance of veterinary services (PVS). The PVS Tool evaluates 36 veterinary regulatory capabilities organized into four major categories: human, physical and financial resources; technical authority and capability; interaction with stakeholders; and access to markets (11). Use of the PVS Tool for external PVS evaluation requires formal written authorization by the OIE. Nevertheless, the tool is available online to give veterinary authorities (and the public) a source for in-depth understanding of what a country’s veterinary services must provide to comply with OIE standards and ensure the absence of disease-causing agents in imported or exported animal products. Veterinary services in each OIE member country are expected to develop their own import regulations based on OIE standards, taking into account the presence or absence of diseases of regulatory concern in their own livestock populations and those of their trading partners.
Qualifications and Certifications
The two largest consumers of animal-derived products for biotechnology are Europe and the United States. So collection, processing, and importation requirements from the USDA and the European Commission (EC) have become de facto veterinary-control standards for the FBS industry. The “US and EU Requirements” box lists examples of such requirements and certifications to be met by veterinary authorities of FBS-exporting countries.
These qualifications and certifications can be met only by countries that have a well-developed animal-health infrastructure — and only through rigorous implementation of regulatory programs.
Comparing Animal Health Regulatory Infrastructures
Most FBS-producing countries are located in the Americas, Europe, and Oceania. Table 1 identifies some of the basic information and infrastructure in 10 countries from those areas of the world that have successful animal-health regulatory programs and services. We refer to the European Union (EU) as if it were one country because it is regulated by a common, unified animal-health regulatory authority: the EC Directorate General (DG) for Food Health and Safety (SANTE). Table 1 lists the name of each country’s animal-health authorities, cattle population statistics, numbers of regulatory veterinarians in the animal-health public sector, animal identification and traceability programs, animal health status, and relative market access to the US and EU markets for beef and bovine serum. Information in Table 1 demonstrates the types of regulatory programs and milestones that must be in place for a country’s industry to access important markets (references at end of article).
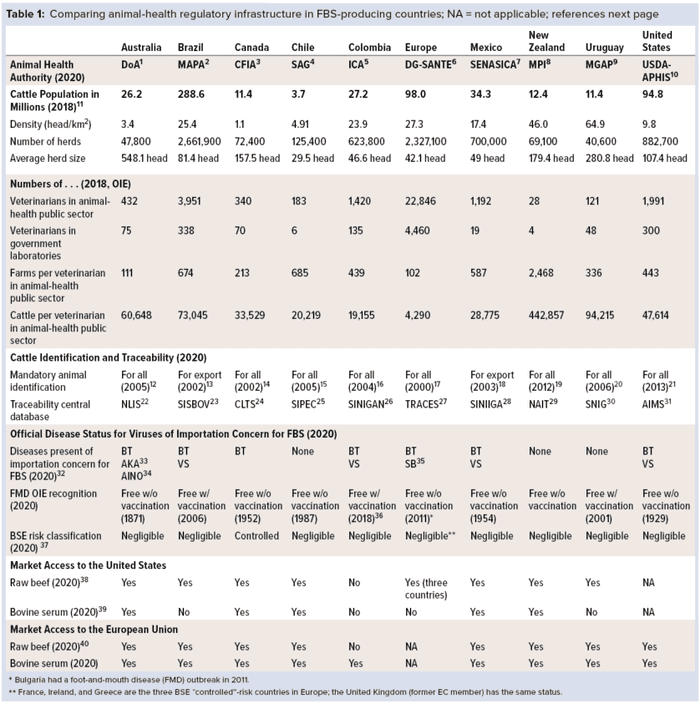
Veterinary Services Authorities: The government entity responsible for veterinary services and animal-health regulatory programs differs from country to country. Table 1 also lists names, acronyms, and links for each such entity in the 10 countries. Animal health normally is part of a ministry of agriculture, livestock, health and food safety, or primary industries. Names of those authorities responsible for animal health in each country have become acronyms used to promote the good reputation associated with the integrity and reliability of their services.
Cattle Population Statistics: The cattle population/density, total number of herds, and average size of herds in each country (Table 1) all are factors that play an important role in how animal health programs are organized and managed by animal-health authorities. Some countries are characterized by having more intensive cattle operations (e.g., Chile and EU, with averages of 30 and 42 animals per herd, respectively), whereas other countries are characterized by their extensive beef-cattle operations (e.g., Australia averaging 548, Uruguay 280, and Brazil 81 animals per herd). Different management approaches and resources are needed to address the differences. Health problems may well be detected more quickly in intensive operations and small–herd-size farms where cattle are seen daily by their owners. In countries characterized by extensive operations where cattle roam free over large tracts of land, health problems are more likely to go undetected for longer periods because the animals might be seen only a few times each year.
Number of Regulatory Veterinarians in Animal-Health Public Sector: The number of veterinarians in the animal-health public sector is another indicator of a country’s veterinary-service abilities to carry out and supervise the many regulatory activities involved in disease-control activities and maintaining export markets. Those include animal identification and traceability, animal movement control, meat inspection, animal welfare monitoring, risk analysis, border protection, and disease surveillance and diagnosis.
The export of bovine serum requires a sufficient number of government-employed veterinarians to be able to verify that requirements of importing countries have been implemented. Such activities might include confirming the country where animals were raised and slaughtered, collecting blood in a government-approved facility and from carcasses that pass antemortem and postmortem inspections, and so on (as listed in the “US and EU Requirements” box). Table 1 also lists the number of veterinarians employed by each government in its animal-health public sector.
According to information provided by each country to the OIE, the number of cattle per veterinarian in the public sector ranges from a high of 443,000 in New Zealand to a low of 4,300 in EU. South American countries range from an average of 20,000 for Chile and Colombia to 94,000 for Uruguay. Note that these numbers do not include consideration of additional private-practice veterinarians who are trained and accredited to perform limited regulatory veterinary inspections and animal disease-control activities on behalf of their governments.
Animal Identification and Traceability: The ability of a country to detect, control, and eradicate cattle diseases is related directly to its ability to identify individual animals and trace and control their movements. Animal traceability systems include registration of livestock premises, declaration of animal inventory on farms, official animal identification, animal-movement control systems, and traceability information database systems. Table 1 provides links to both the cattle-identification program and traceability database used in each of the 10 countries.
Animal-Health Status: To access international markets, FBS-producing countries must be free of diseases that are considered to be of importation concern: e.g., rinderpest, peste des petits ruminants, Rift Valley fever, and foot-and-mouth disease (FMD). The presence of other diseases such as bluetongue, Akabane, Schmallenberg, and vesicular stomatitis also could necessitate preentry barrier treatments (e.g., gamma irradiation, heat, or pH adjustment) or testing upon arrival. Table 1 indicates which diseases of importation concern are present in 10 countries. Australia has the most (three) diseases of importation concern for FBS, whereas New Zealand, Uruguay, and Chile have the least (none). The remaining countries have either one or two diseases of importation concern.
Besides the viruses of importation concern, all FBS must be tested and/or treated for those that are known to be present in all bovine populations around the world (12). For those pathogens, all countries present some level of disease risk. FBS-exporting countries must have in place highly reliable disease prevention, detection, reporting, control, and eradication programs that provide accurate information to the OIE and potential trading partners. Such programs can be successful only with effective cattle identification, traceability, and movement control programs in place.
Bovine spongiform encephalopathy (BSE) continues to be of regulatory concern for some importing countries, even though the OIE declared in 2007 that bovine blood and blood products pose no risk for transmitting it when proper stunning methods are used at slaughter (13). That determination has been endorsed by the World Health Organization WHO (14), USDA (15), and US Food and Drug Administration (FDA) (16). Nobel laureate Stanley Prusiner (17) has stated that cases of spontaneous BSE are to be found at low rates in any cattle population, just as Creutzfeldt-Jakob disease (CJD) is found in all human populations worldwide. Today many regions have reported such cases of spontaneous BSE: e.g., Brazil, Canada, Europe, and the United States. Other cattle-producing countries (e.g., Australia, New Zealand, and many others) that have reported no cases of spontaneous BSE might attribute their lack of reported cases to their extensive-style cattle operations and/or less sensitive surveillance systems for detecting such cases.
Market Access of Beef and Bovine Serum: The ability to access, expand, and retain international markets for beef exports is one important indicator of success and integrity in the animal-health and veterinary public-health systems of a country. Table 1 shows that nine of the 10 listed countries have access to both the US and EU markets for their beef and serum. The only exception is Colombia, which was subject to a temporary beef ban in Europe because of an FMD outbreak in 2017–2018. As of 5 February 2020, that country has regained full recognition by the OIE of its FMD-free status with widespread vaccination.
 Sources You Can Depend On
Sources You Can Depend On
Qualifications and certifications to be met by FBS-producing countries require well-developed animal-health infrastructure and rigorous implementation of regulatory programs. The OIE standards for veterinary services and PVS Tool provide stakeholders with an in-depth examination of the high expectations for a country’s veterinary services to ensure the absence of disease-causing agents in imported and exported animal products.
Countries that are qualified to export FBS to the United States and Europe have met high standards and stringent requirements relating to their animal-health status and veterinary regulatory infrastructures. Despite the widely varying characteristics and conditions among those countries, the animal-health regulatory infrastructure of every FBS-exporting country is tailor-made to meet the OIE’s international standards and the import requirements of their trading partners. So there is no basis for claims that the regulatory infrastructure of some FBS-exporting countries is superior to that of others.
Serum is serum regardless of its country of origin — when it is processed in compliance with all relevant quality control (QC) standards and regulatory requirements. “Serum saves lives,” indeed, and must be available in abundant supply and at affordable prices to researchers and biotechnology companies all over the world.
References
1 Besser L, Cronau P, Baines R. Bovine Blood Products Illegally Smuggled into Australia in International Conspiracy. ABC News 2 July 2018; https://www.abc.net.au/news/2018-07-02/international-conspiracy-smuggle-blood-products-into-australia/9932156.
2 Recall Number Z-1735-2013: Class 2 Device Recall GE Healthcare/PAA Healthcare. US Food and Drug Administration: Rockville, MD, 15 July 2013; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRes/res.cfm?ID=117863.
3 Köppelle W. Unbekannte Zusätze: Fetales Kälberserum (FBS) im Zwielicht. Hat die Österreichische Firma PAA Laboratories Jahrelang Falsch Deklarierte Nährmedien Verkauft, Ohne Ihre Kunden Darüber in Kenntnis Zu Setzen? Laborjournal 11 September 2013; https://www.laborjournal.de/editorials/770.lasso.
4 Proposed Rule: Importation of Fetal Bovine Serum. US Fed. Reg. 59(38) 1994; https://www.govinfo.gov/content/pkg/FR-1994-02-25/html/94-4326.htm.
5 Resolution No. 65: Importation of Fetal Bovine Serum: USDA Response. US Animal Health Association: Reno, NV, 2007; http://www.usaha.org/upload/Resolution/resolution65-2007.pdf.
6 International Serum Industry Association. Serum Sourcing. USAHA Meeting: Kansas City, MO, 2018: 18; https://www.usaha.org/upload/Committee/BiologicsBiotech/ISIA2018.pdf.
7 Hawkes PW, Nielsen OB, Wintgens M. Fetal Bovine Serum: Country of Origin, Geographic Relevance, and Labeling. BioProcessing J. 18, 2019; https://doi.org/10.12665/J18OA.Hawkes.0819.
8 Murray JA, Versteegen RJ. Fetal Bovine Serum: Geographical Origin and International Trade. BioProcessing J. 18, 2019; https://doi.org/10.12665/J18OA.Murray.
9 Terrestrial Animal Health Code. World Organization for Animal Health: Paris, France, 2019; https://www.oie.int/standard-setting/terrestrial-code/access-online.
10 Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. World Organization for Animal Health: Paris, France, 2019; https://www.oie.int/standardsetting/terrestrial-manual/access-online.
11 OIE PVS Pathway. OIE Tool for the Evaluation of Performance of Veterinary Services. World Organization for Animal Health: Paris, France, 2019; https://www.oie.int/fileadmin/Home/eng/Support_to_OIE_Members/docs/pdf/2019_PVS_Tool_FINAL.pdf.
12 Testing Requirements for Extraneous Viruses in Animal Products Used in the Production of Biologics. 9 CFR Part 113, January 2012: 46–53; https://www.govinfo.gov/content/pkg/CFR-2000-title9-vol1/pdf/CFR-2000-title9-vol1-part113.pdf.
13 Chapter 11, Article 11.4.27. Bovine Spongiform Encephalopathy. Terrestrial Animal Health Code. World Organization for Animal Health: Paris, France, 2019; http://www.oie.int/index.php?id=169&L=0&htm¬file=chapitre_bse.htm.
14 Guidelines on Transmissible Spongiform Encephalopathies in Relation to Biological and Pharmaceutical Products. World Health Organization: Geneva, Switzerland 2003; https://www.who.int/immunization_standards/vaccine_regulation/BLOOD_TSE_2003.pdf.
15 Bovine Spongiform Encephalopathy: Importation of Bovines and Bovine Products. US Fed. Reg. 78(233) 2013: 72979–73007; https://www.govinfo.gov/content/pkg/FR-2013-12-04/html/2013-28228.htm.
16 Use of Materials Derived from Cattle in Human Food and Cosmetics. US Fed. Reg. 81(53) 2016: 14718–14732; https://www.gpo.gov/fdsys/pkg/FR-2016-03-18/pdf/2016-06123.pdf.
17 Prusiner, SB. Nobel Lecture: Prions. Proc. Natl. Acad. Sci. USA 95, November 1998: 13363–13383; https://doi.org/10.1073/pnas.95.23.13363.
References: Table 1
1 Department of Agriculture; http://www.agriculture.gov.au.
2 Ministry of Agriculture, Livestock, and Food Supply; http://www.agricultura.gov.br.
3 Canadian Food Inspection Agency; https://www.inspection.gc.ca/eng/1297964599443/1297965645317.
4 Agriculture and Livestock Service; http://www.sag.cl/ambitos-de-accion/pecuaria.
5 Institute of Agriculture and Livestock; http://www.ica.gov.co.
6 European Commission, Directorate General, Health and Food Safety; http://ec.europa.eu/dgs/health_food-safety.
7 National Service of Agriculture and Livestock Health, Food Safety and Quality; https://www.gob.mx/senasica.
8 Ministry of Primary Industries; https://www.mpi.govt.nz.
9 Ministry of Livestock, Agriculture, and Fisheries; http://www.mgap.gub.uy.
10 Department of Agriculture — Animal and Plant Health Inspection Services; https://www.aphis.usda.gov/aphis/home.
11 World Organization for Animal Health (OIE); https://www.oie.int/en/animal-health-in-the-world/world-animal-health.
12 Bowling MD, et al. Identification and Traceability of Cattle in Selected Countries Outside of North America. Prof. Animal Sci. 24, 2008: 287–294; http://www.nationalaglawcenter.org/wp-content/uploads/assets/linkstorage/cattleid-outside.pdf.
13 Muinos V, et al. The Brazilian Bovine Traceability System: A Critical Appraisal. Est. Soc. Agricultura 14(1) 2006; http://socialsciences.scielo.org/pdf/s_esaa/v2nse/scs_03.pdf.
14 Lawrence JD, et al. Lessons Learned from Canadian Cattle Industry, National Animal Identification, and The Mad Cow. Iowa State University: Ames, IA, October 2003. https://www.card.iastate.edu/products/publications/pdf/03mrp7.pdf.
15 Decree 7.219: Creation of Livestock Information System. Government of Chile, Agriculture and Livestock Service: 28 December 2005; http://www.sag.cl/ambitos-de-accion/programa-oficial-de-trazabilidad-animal/1689/normativas?field.
16 Official Diary 45.714. Law 914 of 2004. Government of Colombia: 27 October 2004; http://www.avancejuridico.com/actualidad/documentosoficiales/2004/45714/l0914004.html.
17 EC Regulation 1760/2000. Establishing a System for the Identification and Registration of Bovine Animals and Regarding the Labelling of Beef and Beef Products and Repealing Council Regulation (EC) No 820/97. Off. J. L. 204, 2000: 1–10; http://data.europa.eu/eli/reg/2000/1760/oj.
18 Rule 001-SAG/GAN: National Identification System for Bovines and Beehives. Government of Mexico Official Diary 29 May 2015; http://www.dof.gob.mx/nota_detalle.php?codigo=5394324&fecha=29/05/2015.
19 New Zealand Legislation: National Animal Identification and Tracing (NAIT) Act 2012; https://www.legislation.govt.nz/act/public/2012/0002/35.0/DLM3430220.html.
20 Law 17.997: Animal Identification and Registration System. Government of Uruguay, 8 August 2006; https://www.snig.gub.uy/files/ley-17-997-sistema-de-identificacion-y-registro-animal-4546?es.
21 USDA: Animal Disease Traceability. Title 9, US Code Fed. Reg. Part 86; https://ecfr.io/Title-09/pt9.1.86.
22 Australian National Livestock Identification System (NLIS); https://www.animalhealthaustralia.com.au/what-we-do/biosecurity-services/national-livestock-identification-scheme.
23 Brazil System of Individual Identification of Bovines and Buffalos (SISBOV); http://www.agricultura.gov.br/assuntos/sanidade-animal-e-vegetal/saude-animal/rastreabilidade-animal.
24 Canadian Livestock Traceability System (CLTS); http://support.canadaid.ca.
25 Chile Livestock and Animal Traceability Information System (SIPEC); http://www.sag.gob.cl/ambitos-de-accion/acceso-directo-sipecweb.
26 Colombia National Bovine Identification and Information System (SINIGAN); https://www.fedegan.org.co/programas/sinigan.
27 European Trade Control and Expert System (TRACES); http://ec.europa.eu/food/animals/traces/index_en.htm.
28 Mexico National System for Individual Identification of Livestock (SINIIGA); https://www.siniiga.org.mx.
29 New Zealand National Animal Identification and Tracing (NAIT) System; https://www.mpi.govt.nz/growing-and-harvesting/livestock-and-animal-care/national-animal-identification-and-tracing.
30 Uruguay National Livestock Information System (SNIG); https://www.snig.gub.uy/portal/hgxpp001.aspx.
31 US Animal Identification Management System (AIMS); https://vsapps.aphis.usda.gov/aims.
32 World Animal Health Information Database (WAHIS) Interface. OIE: Paris, France; https://www.oie.int/wahis_2/public/wahid.php/Wahidhome/Home/indexcontent/newlang/en.
33 Center for Food Security and Public Health (CFSPH), Institute for International Cooperation in Animal Biologics. Akabane Disease Factsheet. Iowa State University: Ames, IA, January 2018; http://www.cfsph.iastate.edu/Factsheets/pdfs/akabane.pdf.
34 Center for Food Security and Public Health (CFSPH), Institute for International Cooperation in Animal Biologics. Aino Disease Factsheet. Iowa State University: Ames, IA, January 2018; http://www.cfsph.iastate.edu/Factsheets/pdfs/aino_disease.pdf.
35 Technical Factsheet: Schmallenberg Virus. OIE: Paris, France, April 2017; https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/A_Schmallenberg_virus.pdf.
36 Recovery of “FMD Free Zone Where Vaccination is Practised” Status: Colombia. OIE: Paris, France, 5 February 2020; https://www.oie.int/en/animal-health-in-the-world/official-disease-status/fmd/suspensionreinstatement-of-status.
37 Bovine Spongiform Encephalopathy (BSE). OIE: Paris, France; https://www.oie.int/animal-health-in-the-world/official-disease-status/bse/list-of-bse-risk-status.
38 Food Safety and Inspection Service (FSIS). Eligible Countries and Products. USDA: Washington, DC, 2020; https://www.fsis.usda.gov/wps/portal/fsis/topics/international-affairs/importing-products/eligible-countries-products-foreign-establishments/eligible-countries-and-products.
39 Veterinary Services Notice 98-05: Ruminant Serum Import Requirements. USDA: Washington, DC, 19 March 1998.
40 EC Regulation 206/2010. Laying Down Lists of Third Countries, Territories or Parts Thereof Authorized for the Introduction into the European Union of Certain Animals and Fresh Meat and the Veterinary Certification Requirements. Off. J. L., 12 March 2010; https://eur-lex.europa.eu/eli/reg/2010/206/oj.
Ole Bødtker Nielsen, MSc, is chief executive officer of Biowest in Nuaillé, France; Marc Wintgens, BSc, is president of the European Serum Products Association (ESPA) in Brussels, Belgium; and corresponding author Percy W. Hawkes, DVM, is an independent animal-health regulatory affairs consultant through Hawkes Consulting LLC, 905 South 2300 East, Springville, UT 84663; 1-801-919-9082.










