September 2020 Featured Report
For a host-cell system to generate high yields of recombinant proteins and other entities, cells must be derived from optimized and stable cell lines. However, cell line development (CLD) can be tedious and time-consuming work, and every stage in the CLD workflow has its limitations and challenges. Researchers are creating advanced strategies and tools to overcome those challenges, especially for complex biologics such as bispecific antibodies (BsAbs) and difficult-to-express (DTE) proteins. Online presentations from the CLD track of the BioProcess International Europe conference, which was held 13–17 July 2020, addressed current implementations of high-throughput screening, synthetic biology, genetic engineering, next- generation sequencing, and automated analysis. And new approaches are helping scientists to study genes that are directly related to cell production, quality, and viability.
Current Trends
Monday’s presentations began with two keynote addresses, the first of which focused on current CLD tr...
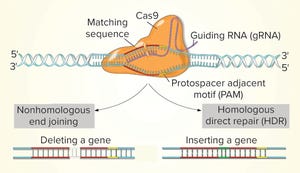
Figure 1: CRISPR-Cas9 (www.stockadobe.com)
While the role of biologics in treating human diseases has evolved dramatically over the past decade, so has genetic engineering. Rational genetic engineering to enhance biotherapeutic proteins has become a reality catalyzed by publication of the genome sequences of multiple Chinese hamster ovary (CHO) cell lines. Novel “designer” CHO cells modulate posttranslational modifications (PTMs) of recombinant proteins by genome editing, and it is now possible to knock-in or knock-out genes of yeast and mammalian cells precisely (within one DNA base pair), quickly, and efficiently. Eventually, such techniques will be used in the development of cost-effective recombinant therapeutic proteins.
The gene-editing tool based on bacterial clustered regularly interspaced short palindromic repeats (CRISPR)–CRISPR-associated protein 9 (Cas9) has improved genome editing dramatically, making it faster, easier, less expensive, and more efficient than before. This system needs only on...
Cell cultures derived from mammalian and bacterial cell lines are the conventional production systems in bioprocessing. But they also have their limitations. Media for mammalian cultures in particular are notoriously expensive, and traditional cell cultures can be highly sensitive to growing conditions. During the late 1980s and into the 1990s, plants and plant-derived cell cultures were introduced as alternative cell-culture systems (
1
,
2
). Although transgenic plants (genetically modified) once looked promising in the early 2000s, the cost and manufacturing complexity of such systems were hurdles that proved to be too high to negotiate, so research in transgenic plants faded. Nonetheless, progress had been made in using plant cell cultures for producing antibodies and proteins (
3
). In the past 10 years, plant cells increasingly have been considered as cost-effective expression systems for recombinant and animal-free proteins, emergency vaccines, immunotherapies, and other products that could benefi...
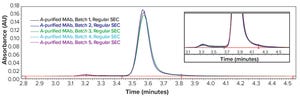
Figure 1: Regular size-exclusion chromatographic (SEC) analysis (pH 6–7) of monoclonal antibody (MAb) products purified by protein A affinity chromatography from batches 1–5
Therapeutic monoclonal antibodies (MAbs) mostly are manufactured using bioengineered mammalian cells cultured in a bioreactor for two to three weeks. High temperatures and an altered redox environment may compromise the quality of MAbs produced (e.g., fragmentation, truncation), as can the presence of proteases, reductases, and other chemicals released from dead cells. Thus, it would be valuable to establish analytical methods that can help cell culture groups monitor immunoglobulin G (IgG) product integrity in real time during a bioreactor run, especially during cell-line development and process scale-up.
In the early years of bioprocessing, product IgG titers usually were low, and animal serum often was included in culture media. That made direct analysis of IgG quality impractical. In the past 10–20 years, however, use of the anima...