Delivery Technology Reenergizes DNA Drug Development
In vivo delivery of DNA-based biopharmaceutical agents encoding proteins of interest (“DNA drugs”) offers a means for the production of protein by target regions of tissue in a subject. This product class derives activity from an ability to induce sustained endogenous protein expression from recipients’ own cells. These unique characteristics are favorable for multiple applications, several of which are now in clinical testing.
Therapeutic Proteins: DNA drugs encoding autologous therapeutic proteins could serve as an alternative to long-term therapy based on administration of recombinant protein-based drugs. By enabling sustained production and secretion of protein (lasting up to weeks or months) from a target tissue with just a single administration, DNA drugs exhibit potential advantages over the frequent injections required to achieve and maintain therapeutic levels of a protein of interest administered as a protein-based drug. Increased convenience and cost effectiveness come from a reduction in the number of required administrations. Without the transient, high-level protein concentrations that occur immediately after dosage of protein-based drugs, DNA drugs may aso reduce potential adverse events.
PRODUCT FOCUS: DNA-BASED BIOPHARMACEUTICALS
PROCESS FOCUS: DRUG DELIVERY
WHO SHOULD READ: FORMULATIONS AND PRODUCT DEVELOPMENT PERSONNEL, PROJECT MANAGERS
KEYWORDS: GENE THERAPY, MEDICAL DEVICES, COMBINATION PRODUCTS, VACCINES
LEVEL: INTERMEDIATE
This approach is currently under clinical investigation for a variety of applications including intratumoral expression of immunomodulatory cytokines and locoregional production of proangiogenic growth factors in ischemic tissue (1,2,3). Substantial data in animals also demonstrate that DNA drugs encoding therapeutic proteins such as immunomodulatory cytokines, hematopoietic factors, and endocrine hormones can provide cost-effective alternatives to systemic protein therapy (4).
Vaccines: DNA vaccination uses DNA sequences encoding immunogenic proteins or protein fragments to elicit immune response against a target antigen. DNA vaccines offer several potential advantages in safety and efficacy over conventional immunization methods. Foremost is the ability to elicit broad, antigen-specific cellular and humoral immune responses without the potential safety issues associated with live pathogens or immune-modulating adjuvants (5). The ability to induce long-term expression of an antigen in a subject’s own cells may also be favorable because it reduces the number of immunizations required to achieve target immune response and/or increases the duration of protection achieved.
Figure 1:
SEBASTIAN KAULITZKI (WWW.ISTOCKPHOTO.COM)
From a manufacturing perspective, DNA vaccines are straightforward to produce and have a favorable stability profile. They are also unique in that each consists of the same chemical entity (deoxyribonucleic acid), and they differ primarily in the amino-acid sequence encoded. As a result, resources and processes developed for manufacture and testing of one product should be readily adaptable to subsequent products, thereby reducing the cost, time, and complexity associated with development of novel DNA vaccines or new variants of existing ones. Therefore, DNA vaccination represents a robust platform for rapid, cost-effective vaccine development that could improve existing vaccines and facilitate development of new ones. Human clinical studies are under way in a broad range of indications including cancer, infectious diseases, autoimmune disorders, and allergies (6). Recent regulatory approvals have been granted for DNA vaccines used in several veterinary indications (7).
RNA Interference: Recent in vivo delivery of DNA vectors expressing short-hairpin interfering RNA (shRNA) sequences shows that a single DNA administration can provide long-term down-regulation of target genes through RNA interference (RNAi) (8). This approach compares favorably with more transient down-regulation of gene expression that is achievable through delivery of small interfering RNA (siRNA) sequences themselves. Initial clinical studies of DNA-based RNAi therapy are now under way in patients with chronic viral infection.
Development Prospects: Overall, DNA drugs represent a promising platform for the development of novel products, with the potential to prevent and/or treat numerous conditions. Recent progress made using several DNA drugs in veterinary applications further confirms this potential (7). To date, however, clinical development of novel DNA-based products has been hampered by low and inconsistent induction of desired responses when administered to humans (5, 6, 9).
A significant contributing factor is the inefficient and inconsistent intracellular delivery associated with conventional DNA delivery methods (5, 9). Because the cell nucleus is the site of transcription, a DNA drug must be taken up into cells for its encoded protein to be produced. Improved methods for intracellular delivery would offer the prospect for realizing the significant potential of DNA drugs in a human clinical setting. A device-based approach known as electroporation (EP) mediated DNA delivery is among the most promising methods demonstrating the capacity for enhancing DNA drug delivery and potency by multiple orders of magnitude over conventional injection alone.
Electroporation-Mediated DNA Drug Delivery
EP is a technique for intracellular delivery based on the brief application of electrical fields to target cells (Figure 1). The process induces a transient state of membrane permeability that allows normally impermeant substances present in the extracellular environment to be efficiently taken up into affected cells. Cell viability is maintained when appropriately selected electrical conditions are used, and the cell membranes rapidly restabilize and resume normal function (subject to the activity of the delivered agent).
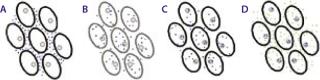
Figure 1: ()
First demonstrated to increase DNA delivery to in vitro cell cultures, EP was adapted for in vivo use (including refinement in application devices and administration conditions) to become a potent method for DNA delivery (10). It was initially believed to increase intracellular uptake by passive diffusion across electrically induced pores formed in affected cell membranes — thus the term electroporation (11). However, the occurrence of membrane pores sufficiently sized to accommodate transmembra
ne diffusion of macromolecules has not been confirmed and is considered unlikely (12). Although the precise mechanisms of EP-mediated in vivo DNA delivery have not been conclusively elucidated, a majority of mechanistic studies support the hypothesis that this technique induces electrophoretic migration of drug agents through destabilized cell membranes (13, 14).
Because the transient cell membrane permeability and electrophoretic migration is a physical response to the application of electrical fields, this technique appears to have broad applicability across different cell types and species (Figure 2). Therefore, it can be adapted for use in different tissues and translate from animal models to a clinical setting. Experience with a variety of animal species has demonstrated that, with appropriate refinement in parameters (e.g., the type of waves and their amplitude, duration, number, and frequency), EP can enhance DNA delivery in a variety of organs/tissues including skeletal muscle, liver, spleen, kidney, brain, blood vessels, bladder, lung, skin, and tumors of various origins — with minimal levels of tissue disruption (10, 13, 15).
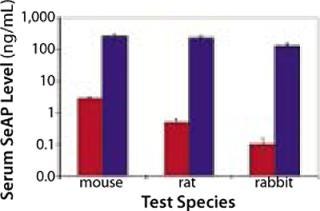
Figure 2: ()
To date, EP-mediated DNA delivery has been evaluated most extensively for local administration in skeletal muscle, skin, and tumors. In each case, the delivery procedure is initiated by administration of DNA to a target region of tissue, most commonly by direct injection. That is followed immediately by application of EP using an array of two or more electrodes configured to propagate electrical fields within tissue at the site of DNA distribution. Because increased intracellular uptake occurs only where DNA is present during EP induction, the essential objective in design of administration procedures and application devices is to ensure “colocalization” of the electrical fields with the site of DNA distribution. As clinical applications of the technology have been envisioned, emphasis has also been placed on refining the EP parameters to minimize tissue disruption as well as discomfort associated with the procedure.
Having demonstrated a capacity to increase uptake and expression of DNA drugs by multiple orders of magnitude over conventional injection (Figures 2 and 3), EP has the potential to facilitate development of DNA drugs for a range of indications requiring locoregional delivery.

Figure 3: ()
EP-based DNA drug delivery provides sustained endogenous expression of therapeutic proteins following a single DNA administration. This technology is positioned to provide a convenient, cost-effective alternative to conventional protein therapies based on frequent injection of a therapeutic protein formulation. Toward this end, DNA-based therapeutic delivery by EP has been shown with a number of different proteins to induce biological responses comparable to those achieved with protein-based drugs (4, 16). Of note is the recent Australian approval of a veterinary DNA drug encoding porcine growth hormone releasing hormone, the first approved DNA product using EP-based delivery (17).
In the DNA vaccine setting, EP dramatically enhances the magnitude of both cellular and humoral immune responses across a broad spectrum of antigens (13, 18). Improved rapidity of response, enhanced dose efficiency, and a reduction in the number of immunizations required to achieve target levels of immunity have also been observed.
Finally, in proof-of-concept studies of DNA-based strategies for RNAi, EP has provided robust, sustained down-regulation of target proteins in multiple cell types (19, 20), suggesting that the technique may be suitable for use in disease indications benefiting from locoregional modulation of protein expression.
By providing a robust, adaptable method for achieving 100- to 1,000-fold enhancement in DNA uptake and expression in a variety of tissue types, EP is well positioned to address the suboptimal clinical potency observed with conventional methods for DNA drug administration.
Device Development and Transition to Clinic
Based on compelling results generated with EP-mediated DNA delivery in animal studies, the technology is now used routinely for localized delivery of DNA drugs in nonclinical research applications. However, the viability of the technology for commercial clinical application will depend on a number of critical factors. Foremost among these are an ability to achieve effective and reproducible delivery that is safe, cost-effective, and well tolerated by the relevant target population.
With several different groups moving forward into clinical testing, these challenges are now being addressed. In addition to comprehensive preclinical safety testing for each individual combination product (including a DNA drug and EP device), key considerations for initiation of clinical studies have included provision of administration procedures and devices that achieve the necessary consistent colocalization within target tissue across heterogeneous subject populations (18). As use of this technique expands beyond its initial therapeutic application to metastatic cancer, greater emphasis has also been placed on tolerability characteristics. Over the longer term, the commercial feasibility of EP will depend on enhancing activity of DNA drugs to a level sufficient to justify the costs and complexity of combination drug–device products.
To date, seven human clinical studies using EP-mediated DNA delivery have been publicly announced (Table 1), including trials of DNA drugs encoding therapeutic proteins as well as DNA vaccines.
Table 1: Human clinical studies of electroporation-mediated DNA drug delivery

Table 1: Hu man clinical studies of electroporation-mediated DNA drug delivery ()
The first clinical study of DNA delivery with EP was initiated in 2004 at the Moffitt Cancer Center to evaluate intratumoral delivery of DNA encoding a potent immunomodulatory cytokine (interleukin-12, IL-12) in patients with metastatic melanoma. The IL-12 DNA is delivered into surface-accessible metastatic lesions using MedPulser EP technology developed by Inovio Biomedical Corp (San Diego, CA). The device involves a two-step administration procedure: DNA is administered into a tumor by injection, followed by manual insertion and activation of an array of exposed electrodes into tumor tissue judged by a physician operator to be the site of agent distribution. An additional clinical trial using the same device for intratumoral DNA delivery includes a multicenter study sponsored by Vical, Inc. (San Diego, CA) administering DNA encoding another immunostimulatory cytokine (interleukin-2, IL-2), also in patients with metastatic melanoma.
In addition to those intratumoral applications, the MedPulser device is also being used in two studies of intramuscular DNA immunization delivery. These include delivery of a DNA vaccine encoding the carcinoembryonic antigen (CEA) with the HER2 epidermal growth factor receptor in cancer patients and a DNA vaccine encoding antigens from the hepatitis C virus in chronically infected patients.
For manually controlled EP administration procedures (exemplified by the MedPulser device), the effectiveness of that two-step approach for colocalization of DNA drug and electric fields is based substantially on the skill of an operator. So it requires extensive user training and is probably subject to considerable variability across heterogeneous patient populations. Although potentially suitable for use by highly trained physicians for intratumoral delivery in surface-accessible lesions, such an approach is likely to be suboptimal for applications such as intramuscular or intradermal DNA delivery.
Based on those considerations, a consensus is emerging in the field that EP device technologies will be favored that integrate the means for agent administration and electrical field application into a single apparatus. Such integration of all the procedural elements into a single device should facilitate operator training, improve reproducibility, and decrease the risk of false-negative results in clinical trials resulting from improper and/or inconsistent administrations. In addition, device technologies for simple and rapid administration are likely to be favored by patients and health-care personnel alike. Two such integrated device technologies have progressed into human clinical testing.
The first to enter clinical testing was the Twinjector integrated device from Inovio Biomedical Corporation used in a multicenter UK trial for administration of a DNA vaccine encoding an epitope derived from the prostate specific membrane antigen (PSMA) in prostate cancer patients. Designed for intramuscular injection, this device consists of a reusable, hand-held applicator connected to an electrical signal generator. It uses two off-the-shelf syringes, their injection needles serving both as a means for agent administration and as electrodes for EP application. A mechanical gearing mechanism enables operators to simultaneously actuate needle insertion and agent administration. This results in distribution of DNA in two columns centered on the injection needle electrodes. Although it has not yet reached the clinical stage, an improved version of the device (the Elgen 1,000 device) combines the same two-needle DNA injector with motorized control over the rate of injection and needle insertion.
The intramuscular TriGrid delivery system (TDS-IM) integrated EP device developed by Ichor Medical Systems (San Diego, CA) is in clinical testing as well. It consists of three components: a pulse stimulator, an integrated applicator, and a single-use application cartridge (Figure 4). In this case, the disposable application cartridge is the only patient-contact component, which minimizes the risk of cross-contamination between subjects. The product is packaged sterile for a single use and is attached to the integrated applicator at the beginning of an administration procedure. The cartridge encloses an array of four electrodes arranged in two interlocking triangles (hence the name TriGrid) around a central injection needle. By aligning the long axis of the diamond-shaped electrode array with the orientation of myofibers in target muscle tissue, the EP-inducing electrical fields will be propagated in a manner consistent with the fluid distribution pattern characteristic of an intramuscular injection. The integrated applicator is a reusable hand-held device that automatically deploys the electrodes and administers DNA, providing user-independent control over the rate and site of administration. It connects to the pulse stimulator, which monitors the procedure for safety and generates the electrical fields.
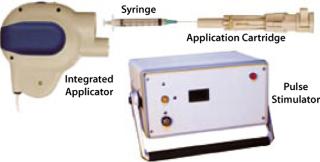
Figure 4: ()
The integrated, automated design of the TDS-IM system enables operators to apply the entire procedure in a few seconds while ensuring that EP is applied consistently to tissues at the site of DNA distribution. Use of a deployable injection needle and electrodes with an automated stick shield that engages at conclusion of a procedure minimizes visualization and exposure to sharps. This device is currently in clinical testing with a therapeutic DNA vaccine encoding a melanosomal antigen in melanoma patients who are at high risk for recurrent disease. It is also being evaluated in a placebo-controlled comparative study of EP delivery and conventional intramuscular injection for delivery of a prophylactic HIV vaccine. Indicative of the progress that has been made in characterizing safety and tolerability of the procedure, this final study represents the first EP-mediated DNA delivery in healthy human subjects.
Outlook
The enhanced DNA drug potency observed with EP-mediated DNA delivery provides the basis for early phase clinical trials in a variety of indications. Combining an acceptable safety profile in initial therapeutic indications with improvements in device technology has enabled initiation of a prophylactic DNA vaccine study. Interim results from the IL-12 and PSMA trials have demonstrated induction of gene expression and/or downstream biological response. The recent approval of a veterinary DNA drug delivered using EP provides further support for the utility of this technology.
Given the rapid progress in this field since initiation of the first clinical trials in 2004–2005, a host of new developments are expected over the coming 18–24 months. Data collected from ongoing clinical investigations will provide valuable insight as to whether current EP technology allows the compelling results from animal models to be extrapolated into humans — and whether the benefits of EP are sufficient to justify its costs. It is also expected that at least 10 more clinical trials of EP-mediated DNA drug delivery will be initiated, significantly expanding the range of indications under evaluation.
Other EP technologies currently in preclinical testing are expected to reach the clinical sta
ge, expanding the routes of administration and types of devices available for investigation of EP-mediated DNA administration. In addition to the ongoing opportunities in DNA-based protein drug and vaccine delivery, preclinical progress with EP-based delivery of other novel classes of DNA drugs (including vectors for RNAi) may further broaden the range of clinical applications for this technology.
Taken together, recent advances in EP-based delivery provide a basis for cautious optimism that the technology may help realize the long-awaited promise of DNA drugs.
REFERENCES
1.) Mahvi, DM. 2007. Intratumoral Injection of IL-12 Plasmid DNA: Results of a Phase I/IB Clinical Trial. Cancer Gene Ther. 14:717-723.
2.) O’Malley, BW. 2005. Combination Nonviral Interleukin-2 Gene Immunotherapy for Head and Neck Cancer: From Bench Top to Bedside. Laryngoscope 115:391-404.
3.) Gaffney, MM. 2007. Cardiovascular Gene Therapy: Current Status and Therapeutic Potential. Br. J. Pharmacol. 152:175-188.
4.) Prud’homme, GJ. 2006. Electroporation-Enhanced Nonviral Gene Transfer for the Prevention or Treatment of Immunological, Endocrine and Neoplastic Diseases. Curr. Gene Ther. 6:243-273.
5.) Ulmer, JB, B Wahren, and MA. Liu. 2006. Gene-Based Vaccines: Recent Technical and Clinical Advances. Trends Mol. Med. 12:216-222.
6.) Liu, MA, and JB. Ulmer. 2005. Human Clinical Trials of Plasmid DNA Vaccines. Adv. Genet. 55:25-40.
7.) Salonius, K. 2007. The Road to Licensure of a DNA Vaccine. Curr. Opin. Investig. Drugs 8:635-641.
8.) Romano, PR, DE McCallus, and CJ. Pachuk. 2006. RNA Interference-Mediated Prevention and Therapy for Hepatocellular Carcinoma. Oncogene 25:3857-3865.
9.) Wolff, J. 2005. Non-Viral Approaches for Gene Transfer. Acta Myol. 24:202-208.
10.) Somiari, S. 2000. Theory and In Vivo Application of Electroporative Gene Delivery. Mol. Ther. 2:178-187.
11.) Neumann, E. 1982. Gene Transfer Into Mouse Lyoma Cells By Electroporation in High Electric Fields. Embo. J. 1:841-845.
12.) Sukharev, SI. 1992. Electroporation and Electrophoretic DNA Transfer into Cells: The Effect of DNA Interaction with Electropores. Biophys. J. 63:1320-1327.
13.) Favard, C, DS Dean, and MP. Rols. 2007. Electrotransfer As a Non Viral Method of Gene Delivery. Curr. Gene Ther. 7:67-77.
14.) Satkauskas, S. 2002. Mechanisms of In Vivo DNA Electrotransfer: Respective Contributions of Cell Electropermeabilization and DNA Electrophoresis. Mol. Ther. 5:133-140.
15.) Scherman, D, P Bigey, and MF. Bureau. 2002. Applications of Plasmid Electrotransfer. Technol. Cancer Res. Treat. 1:351-354.
16.) Jaini, R. 2006. Gene-Based Intramuscular Interferon-Beta Therapy for Experimental Autoimmune Encephalomyelitis. Mol. Ther. 14:416-422.
17.) 2008. VGX Animal Health Announces Approval of LifeTide™ SW 5, VGX Pharmaceuticals, Inc., Blue Bell.
18.) Luxembourg, A, CF Evans, and D. Hannaman. 2007. Electroporation-Based DNA Immunisation: Translation to the Clinic. Expert Opin. Biol. Ther. 7:1647-1664.
19.) Magee, TR. 2006. Myostatin Short Interfering Hairpin RNA Gene Transfer Increases Skeletal Muscle Mass. J. Gene Med. 8:1171-1181.
20.) Takahashi, Y, M Nishikawa, and Y. Takakura. 2006. Suppression of Tumor Growth By Intratumoral Injection of Short Hairpin RNA-Expressing Plasmid DNA Targeting Beta-Catenin or Hypoxia-Inducible Factor 1a. J. Control. Rel. 116:90-95.
You May Also Like





