Evaluation of a Novel Peptide-Based Affinity Ligand for Human IgM Purification: Use of an Automated Liquid-Handling System for Rapid Assessment of Binding Kinetics and Capacity
February 4, 2021
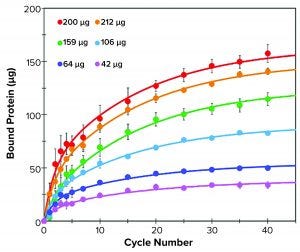
Figure 1: Binding kinetics of different amounts of human IgM to resin M over 40 binding cycles; bound IgM was measured at 1, 2, 3, 4, 5, 7, 10, 15, 20, 25, 30, 35, and 40 binding cycles (filled circles). Data were fitted with a double exponential function (solid lines).
One-step affinity purification of antibodies is a powerful and widely used tool in the biopharmaceutical industry. Although different strategies can be used to purify immunoglobulin isotype G (IgG), the larger antibody isotype IgM has limited options. Human IgM is the largest antibody and primarily exists as a pentamer in the bloodstream (1). IgM molecules exhibit higher avidity than other antibodies do and serve as essential activators for the complement cascade (1–3). Approaches to IgM purification with hydroxyapatite and ion-exchange (IEX) chromatography can require additional polishing steps (4). Recent introduction of IgM-affinity ligands has enabled single-step affinity purification, thereby opening therapeutic application opportunities (5).
Below, we describe the use of IMCStips dispersive solid-phase extraction technology to purify IgM antibodies. The method used a novel resin (from LigaTrap Technologies) that is based on a single peptide. For human IgM resin (resin M), the reported capacity in spin-column format is 10.3 ± 0.3 mg/mL of resin with a 98% purity of the eluate (6). The case study reported here used an automated, rapid purification of human IgM across a range of concentrations to determine capacity and binding kinetics in a tip-based format.
Materials and Methods
Chemicals and Reagents: IMCStips containing 10 µL of resin M (from LigaTrap) were provided by IMCS. Human IgM was purchased from ImmunoReagents. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) confirmed IgM purity for the experiment. Vendor-recommended buffers for equilibration and wash buffers were 10 mg/mL adipic acid, 800 mM NaCl at pH 5.8, sample diluent as 50 mg/mL adipic acid, 4.0 M NaCl at pH 5.8, elution buffer as 0.1 M sodium acetate, pH 4.0 and neutralization buffer as 3.0 M Tris base, pH 11.1. LigaTrap provided sample diluent for the binding kinetics experiments.
Equipment: For all experiments, we used a Hamilton Microlab STAR liquid handler equipped with a CO-RE 96-channel multiprobe head (MPH). Protein concentration was measured using a NanoDrop 2000 spectrophotometer. We set an extinction coefficient value of 11.8 mg mL–1 cm–1 (E1% at 280nm) for quantifying human IgM.
Binding Kinetics: Load concentrations of human IgM were varied and diluted in 1× phosphate-buffered saline (PBS) and sample diluent (45 µL) for a final volume of 225 µL. Each load amount was run in triplicate. We tested protein concentration for each set at binding cycles 1, 2, 3, 4, 5, 7, 10, 15, 20, 25, 30, 35, and 40. Before human IgM purification, tips were equilibrated with equilibration buffer for four cycles (aspiration = 30 µL/s, dispense = 15 µL/s). Samples were bound for 40 binding cycles at 200 µL per aspiration (aspiration = 30 µL/s, dispense = 5 µL/s).
Full Method Purification: For purifying human IgM, we used the vendor-recommended buffers except for the elution buffer. The elution buffer used for purifications in IMCStips solid-phase extraction was 0.1 M sodium acetate at pH 3.0. For human IgM purification, we used pure IgM spiked into HCT116/200 media containing 1% fetal bovine serum (FBS). The IgM purification process had three equilibration cycles, 40 binding cycles, two wash steps with five mixing cycles each, and two elution steps with 20 mixing cycles each. We performed all SDS-PAGE analyses using 4–20% gradient Tris-glycine gels.
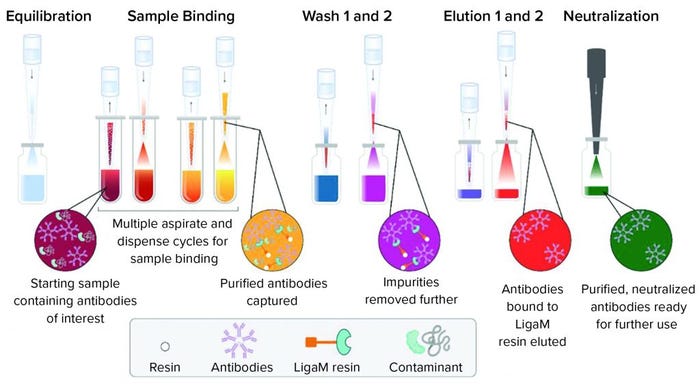
Figure 2: A workflow for purification of human IgM using a tip-based purification approach consists of equilibration of the resin in each tip with mixing cycles, sample binding cycles to allow the target to bind, two wash steps to remove nonspecifically bound material, and two elution steps to collect the target antibody.
Results
Binding Kinetics and Capacity: We determined binding kinetics of 10 µL of resin M (Figure 1) in 300-µL tips using the liquid handler equipped with compressed O-ring expansion (CO-RE) 96-channel MPH. For each sample, we varied load amounts of the respective pure antibody in 225-µL solution and purified over 40 aspiration-dispense cycles (binding cycles). Each binding cycle lasted 48 seconds. We calculated the amount of bound target protein as the difference of protein remaining in the flow-through relative to the starting protein amount. After a selected number of cycles, free protein in solution was determined with absorbance at 280 nm. We fit binding data using a biphasic exponential associative function.
Resin M demonstrates classic biphasic kinetics found in many porous resins. The two kinetics are a fast (surface binding) and slow (pore diffusion-mediated) binding. Binding kinetics are affected by protein concentration, pore size, and analyte size. Additional factors such as protein concentration, relative ligand density, and particle size also influence binding kinetics.
IgM (~960 kDa) is a large molecule that exhibits lower effective diffusivity and is more likely to show slow overall kinetics. An initial rapid binding event occurs over the first five cycles, followed by a slower diffusion-mediated event over the next 35 cycles (Figure 1). At the highest protein load (265-µg IgM), 10 µL of resin was able to capture 150 µg of IgM in 40 binding cycles. In that case, roughly half of the bound material
(~75 µg) was captured in the first five binding cycles.
The diffusion-related binding event is evident when comparing the rates of binding for saturating protein loads (>200 µg) with those of the 106-µg sample. In such cases, the surface sites are likely to be fully occupied, but the low concentration of free protein remaining in the 106-µg sample results in slower binding kinetics to the ligands present in the interior pores. Meanwhile, both saturating conditions (212 µg and 265 µg) exhibited similar binding kinetics to each other for both the fast and slow portions of binding. These results supported the manufacturer’s reported values, with a maximum tip binding capacity of 15 mg/mL resin at 40 binding cycles under saturating conditions.
Full Method Purification: The fully automated high-throughput purification workflow followed a traditional affinity approach of equilibrate, bind, wash, and elute (Figure 2). Purification yields ranged from near quantitative to 56% at saturating protein amounts (>150 µg) (Figure 3a). At the highest protein load (229 µg), 128 µg of purified IgM was recovered. Purification of 91 µg of IgM spiked into spent media showed purity consistent with previous reports from the manufacturer (Figure 3b). Presence of a detectable band was suggestive of FBS copurification. Overall, purification amounts reflected previously reported binding capacities in which LigaTrap was able to purify 12.8 mg of human IgM per mL of resin (6).
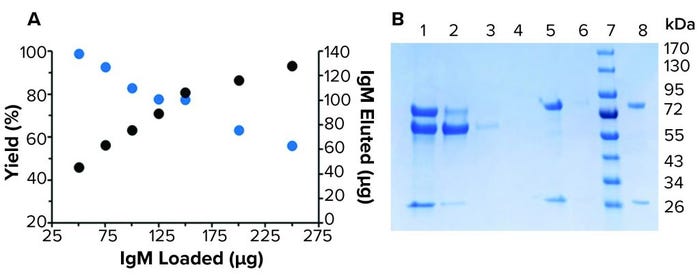
Figure 3: Purification of human IgM from spent HCT116/200 media; (a) purification of various loads of IgM by 10 µL resin M in IMCStips. The yield (blue) ranged 99–56%. The amount of IgM recovered (black) ranged 45–128 µg. (b) A reducing SDS-PAGE related to the purification of 91 µg of IgM in spent media. 1-load, 2-flow-through, 3-wash 1, 4-wash 2, 5-elution 1, 6-elution 2, 7-marker, and 8-IgM standard.
Reducing IgM Bottlenecks
Rapid evaluation of a resin with the dispersive solid-phase extraction indicated similar capacity results provided by LigaTrap for loose resin protocols. Moreover, this workflow was fully automated and could be used to purify 1–96 samples simultaneously within an hour. IgM-based therapeutics represent a rapidly growing industry. The high-throughput, small-scale purification of IgM moieties presented in this work might lead to a significant reduction in bottlenecks associated with antibody purification.
References
1 Grönwall C, Vas J, Silverman G. Protective Roles of Natural IgM Antibodies. Front. Immunol. 3, 2012: 66; https://doi.org/https://doi.org/10.3389/fimmu.2012.00066.
2 Kaveri SV, Silverman GJ, Bayry J. Natural IgM in Immune Equilibrium and Harnessing Their Therapeutic Potential. J. Immunol. 188 (3) 2012: 939–945; https://doi.org/10.4049/jimmunol.1102107.
3 Sharp TH, et al. Insights into IgM-Mediated Complement Activation Based on In Situ Structures of IgM-C1-C4b. Proc. Natl. Acad. Sci. 116(24) 2019: 11900– 11905; https://doi.org/10.1073/pnas.1901841116.
4 Gagnon P. IgM Purification with Hydroxyapatite. BioProcess Int. 12(2) 2014: 40–51; https://bioprocessintl.com/upstream-processing/biochemicals-raw-materials/igm-purification-with-hydroxyapatite-349783.
5 Reese H, Bordelon T, Shanahan C. Novel Peptoid-Based Adsorbents for Purifying IgM and IgG from Polyclonal and Recombinant Sources. J. Chromatogr. B 1137, 2020: 121909; https://doi.org/https://doi.org/10.1016/j.jchromb.2019.121909.
6 Odeh F, Rozakis S, Bordelon T. The Forgotten Immunoglobulin LigaTrap Technologies and Advancements in IgM Purification. BioProcess Int. 15(8) 2017: 43–45; https://bioprocessintl.com/2017/august-2017/forgotten-immunoglobulin-ligatrap-technologies-advancements-igm-purification.
Carter Mitchell is chief technology officer at Kemp Proteins, LLC. Dana N. Moore is biomanufacturing associate, and Patrick Kates is research scientist at Integrated Micro-Chromatography Systems, Inc. John Stevens is a scientist, and Jae Sly is chief business officer at LigaTrap Technologies, LLC. Corresponding author L. Andrew Lee is chief scientific officer at IMCS; 1-888-560-2073; [email protected].
You May Also Like





